Availability, Accessibility, and Utilization of Diagnostics and Therapeutics for Spontaneous Intracranial Hypotension in Asia
Article information
Abstract
Purpose
Recent advances in imaging techniques have significantly enhanced the diagnosis of spontaneous intracranial hypotension (SIH). However, these developments have been reported mostly in Europe and the United States. This study aimed to evaluate the availability and utilization of diagnostic and treatment modalities for SIH in Asia, through a survey of regional headache specialists.
Methods
A literature search was conducted using PubMed, and members of the Asian Regional Consortium for Headache were contacted. Participants completed a two-step survey evaluating the availability, accessibility, and frequency of SIH diagnostic and treatment methods in their countries and institutions. Descriptive statistics were used to analyze the data.
Results
Twenty physicians from eight countries completed both rounds of the survey. Lumbar puncture, brain magnetic resonance imaging (MRI), and spinal MRI are widely available across Asia, but real-time imaging techniques—such as dynamic computed tomography myelography and digital subtraction myelography—that precisely localize cerebrospinal fluid leaks are less accessible. Blind or semi-targeted epidural blood patches (EBPs) are available at most centers, but are easily accessible in only about half of cases. Surgical interventions are rarely available.
Conclusion
Most diagnostic methods for SIH are available in Asia, despite some regional disparities. The utilization of EBP and surgical interventions remains somewhat limited. This highlights the need for greater awareness and standardization of diagnostic methods in Asia.
INTRODUCTION
Spontaneous intracranial hypotension (SIH) is caused by a spinal cerebrospinal fluid (CSF) leak, leading to serious morbidity and disability.1 The incidence of SIH is estimated to be 3–5 per 100,000 people annually.2,3 Patients with SIH typically experience acute orthostatic headaches along with various cranial nerve-related symptoms such as double vision, tinnitus, imbalance, and cognitive impairment.4,5 Over the past decade, diagnostic methodologies of SIH have been significantly advanced. The use of heavily T2-weighted noninvasive magnetic resonance (MR) myelography has enabled noninvasive identification of spinal longitudinal epidural collections (SLEC) more sensitively than conventional computed tomography (CT) myelography and has facilitated the diagnosis of SIH.6,7 Furthermore, the introduction of real-time imaging techniques such as ultrafast CT myelography and digital subtraction myelography (DSM), has provided better localization of leaking sites. Based on these advancements, a new causative classification of SIH has been introduced.8-10
However, the use of real-time imaging techniques has been reported only in a limited number of centers in a limited number of countries, mainly in Europe and the United States. To determine whether Asia-specific regional issues or unmet needs should be addressed, we aimed to identify the current availability and use of diagnostic and therapeutic modalities for SIH in Asia. For this purpose, we conducted a qualitative survey with Asian SIH researchers and headache specialists.
MATERIALS AND METHODS
1. Ethics approval and consent to participate
This study involved a voluntary survey of medical professionals across multiple institutions and countries. The survey did not involve patient data or sensitive personal information. Given the nature of the study, formal ethics approval was not sought, and all participants responded voluntarily with the understanding that their responses would be used for research purposes. No identifying information is reported in the manuscript.
2. Study design
We invited core members of the Asian Regional Consortium for Headaches (ARCH) who had participated in the ARCH business meetings and were based in Asian countries. We also included clinicians or researchers from under-represented areas, regions, or countries based on recommendations from the ARCH members.
To further supplement country representation, we conducted a literature search of the PubMed database using the following search terms: SIH, CSF leak, intracranial hypotension, CSF hypovolemia, cerebrospinal fluid hypovolemia, low-pressure headache, low CSF volume, and low cerebrospinal fluid pressure. The search terms were combined with Medical Subject Headings (MeSH) and non-MeSH terms using Boolean operators (OR and/or AND). We identified papers in which the corresponding author was based in Asia and contacted those from countries not already represented by ARCH core members. The full details of the search strategy are provided in Supplementary Table 1 (available online), and the selection process for survey participants according to the country of affiliation is provided in Supplementary Table 2 (available online).
3. Survey
Potential participants were invited to respond to a questionnaire to assess the availability of diagnostic and therapeutic methods for SIH in their countries and institutions. In Round 1, the availability of each diagnostic or therapeutic method was surveyed at the national and institutional levels. Diagnostic methods included brain magnetic resonance imaging (MRI) with contrast, lumbar puncture, noninvasive spine MRI without heavily T2-weighted imaging, noninvasive spine MRI with heavily T2-weighted imaging, spine MR myelography with intrathecal gadolinium, ultrafast dynamic CT myelography, conventional CT myelography, DSM, and radioisotope (RI) cisternography. Therapeutic methods include conservative management, blind epidural blood patch (EBP), semi-targeted EBP, targeted EBP, and surgical repair.
In the Round 2 survey, respondents were asked to rate the accessibility of each diagnostic or therapeutic method at both the national and institutional levels. For each method, they rated accessibility using a 4-point scale: easily accessible, somewhat accessible, not easily accessible, and not available. The frequency of use of each method in practice was also surveyed using a 4-point scale: frequently, occasionally, rarely, and never.
In Round 2, we explored the barriers to using specific diagnostics and therapeutics, including ultrafast CT myelography, DSM, targeted EBP, and surgical repair. The participants responded to a multiple-choice question that allowed them to select multiple answers. A full list of response options is provided in the Results section.
4. Epidural blood patch type definition
To avoid confusion regarding EBP types, we adopted the following definitions in this study. These definitions were employed to ensure consistency and clarity throughout the study. Blind EBP refers to a blood patch performed at a specific anatomical site determined by the clinician without spinal imaging. Semi-targeted EBP refers to a blood patch administered at a site suspected of leakage based on MR myelography or conventional CT myelography. The clues used for targeting included not only epidural fluid collections but also findings such as periradicular leaks. Targeted EBP refers to a blood patch performed after localizing the precise CSF leak site using real-time imaging techniques, such as ultrafast dynamic CT myelography or DSM.
5. Statistical analysis
Descriptive statistics were used to analyze the data. Categorical variables are expressed as frequencies and percentages. The analysis primarily focused on the frequency distribution of the responses. All statistical analyses were performed using Microsoft Excel.
RESULTS
1. Study participants
Among the 51 Asian countries, we identified 819 authors from 18 countries through a literature search. After merging the ARCH members’ lists, invitations were e-mailed to 56 physicians from 22 countries. We received responses from 27 physicians from 11 countries: China, Japan, Korea, Malaysia, Mongolia, Singapore, Taiwan, Thailand, Türkiye, Vietnam, and the United Kingdom. One participant from the United Kingdom was excluded from the analysis in Round 1 because the participant’s workplace had changed outside Asia. Figure 1 illustrates the flowchart and the full list of invitations and responses according to Asian countries is provided in Supplementary Table 2 (available online). Participants from 10 countries in Round 1 and eight in Round 2 completed the survey, as shown in Table 1. All respondents were neurologists, except for one neurosurgeon who participated in Round 1.
2. Availability at the national and center level and usage
In Round 1, brain MRI with contrast and lumbar puncture were reported to be available in all 11 countries and 22 center levels. Noninvasive spine MRI with or without heavily T2-weighted imaging was available; 25 of 26 respondents (96.15%) reported that it was available in their nation. There was a notable gap between the national and central levels for ultrafast dynamic CT myelography and DSM. While 18 (69.23%) and 15 respondents (57.69%) guessed that these tests were available in their countries and centers, respectively, only 10 respondents (38.46%) from six centers had both tests available. One center provided only ultrafast dynamic CT myelography, whereas the other provided only the DSM (Figure 2A). Regarding therapeutic methods (Figure 2B), conservative management was available in all 11 nations and 22 centers. Blind EBP, semi-targeted EBP, and targeted EBP were reported to be available at the national and central levels as follows: 22 (84.62%) and 18 (69.23%) for blind EBP, 24 (92.31%) and 20 (76.92%) for semi-targeted EBP, and 21 (80.77%) and 16 (61.54%) for targeted EBP. Surgical repair was the least available option, with 19 (73.08%) and 11 respondents (42.31%) indicating availability at the national and central levels, respectively.
3. Accessibility at the national level
The accessibility of various diagnostic and therapeutic methods at the national level in the eight countries is summarized in Figure 3. Brain MRI with contrast, lumbar puncture, and noninvasive spine MRI without heavily T2-weighted imaging were easily accessible in most countries (N=7, 87.5%). Noninvasive MR myelography with heavily T2-weighted imaging and conventional CT myelography was somewhat accessible in five (62.5%) and four countries (50.0%), respectively. Only one country (Taiwan) reported that noninvasive MR myelography with heavily T2-weighted imaging is easily accessible. Ultra-fast dynamic CT myelography and DSM are either not easily accessible or are unavailable in all countries. Spinal MR myelography with intrathecal gadolinium is unavailable in most countries.
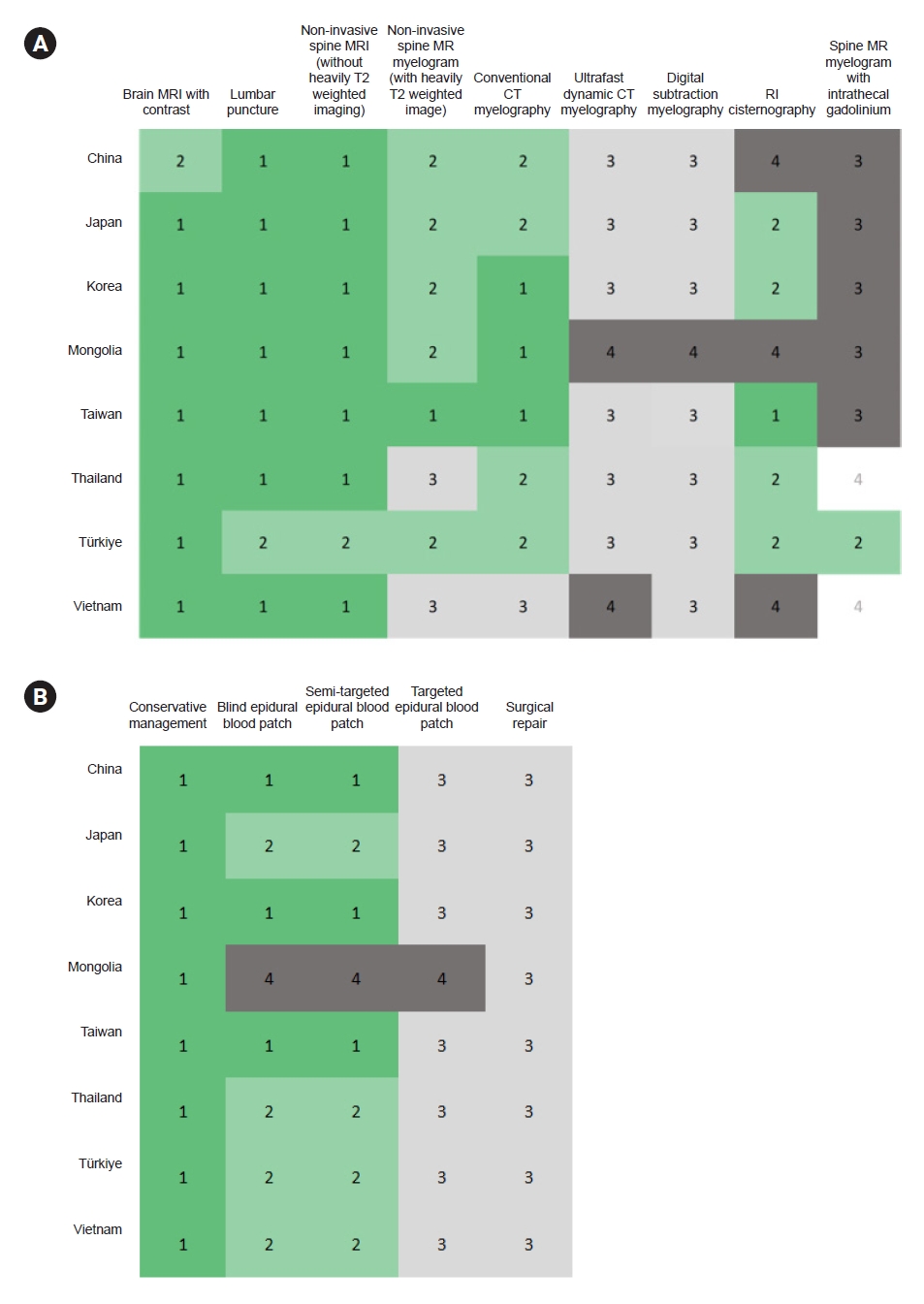
Heat map of (A) diagnostic and (B) therapeutic method accessibilities nationally in Asia. The accessibility of each method was rated with a 4-point scale—1) easily accessible, 2) somewhat accessible, 3) not easily accessible, and 4) not available—except for spine magnetic resonance myelography with intrathecal gadolinium, which was rated using the following scale: 1) available and approved; 2) available but not approved, can be used off-label; 3) not approved and not available; and 4) do not know.
MRI, magnetic resonance imaging; MR, magnetic resonance; CT, computed tomography; RI, radioisotope.
Conservative management is accessible in all countries. Blind or semi-targeted EBP were quickly or somewhat accessible in seven countries (87.5%). Nevertheless, the targeted EBP was not easily accessible in all the seven countries where EBPs were available. Surgical repair is also not readily accessible in any of the eight countries. This pattern shows limited access to techniques that can identify and seal the exact leakage site in most Asian countries despite their technical availability.
4. Accessibility at the institutional level and usage
Across the 16 centers, brain MRI with contrast, lumbar puncture, and noninvasive spinal MRI without heavily T2-weighted imaging were rated as easily accessible in 15 centers (93.75%). Noninvasive spine MR myelography with heavily T2-weighted imaging and conventional CT myelography was easily accessible in 10 (62.50%) and nine centers (56.25%), respectively. Ultrafast dynamic CT myelography was not easily accessible, but it was somewhat accessible in four centers (25.00%). The DSM was easily accessible in two (12.50%) and slightly accessible in seven centers (43.75%). RI cisternography was accessible in six (37.50%). Spine MR myelography with intrathecal gadolinium was off-label in four centers (25.00%) (Figure 4A). Conservative management was easily accessible at all centers (100%). Although blind and semi-targeted EBP were available in 14 centers (87.50%), they were easily accessible in only eight centers (50.00%). Furthermore, targeted EBPs were easily accessible in only three centers (18.75%) and were somewhat accessible in another three centers (18.75%). Surgical repair was the least accessible treatment, with only one center (6.25%) finding it easily accessible, and three centers (18.75%) finding it somewhat accessible (Figure 4B).
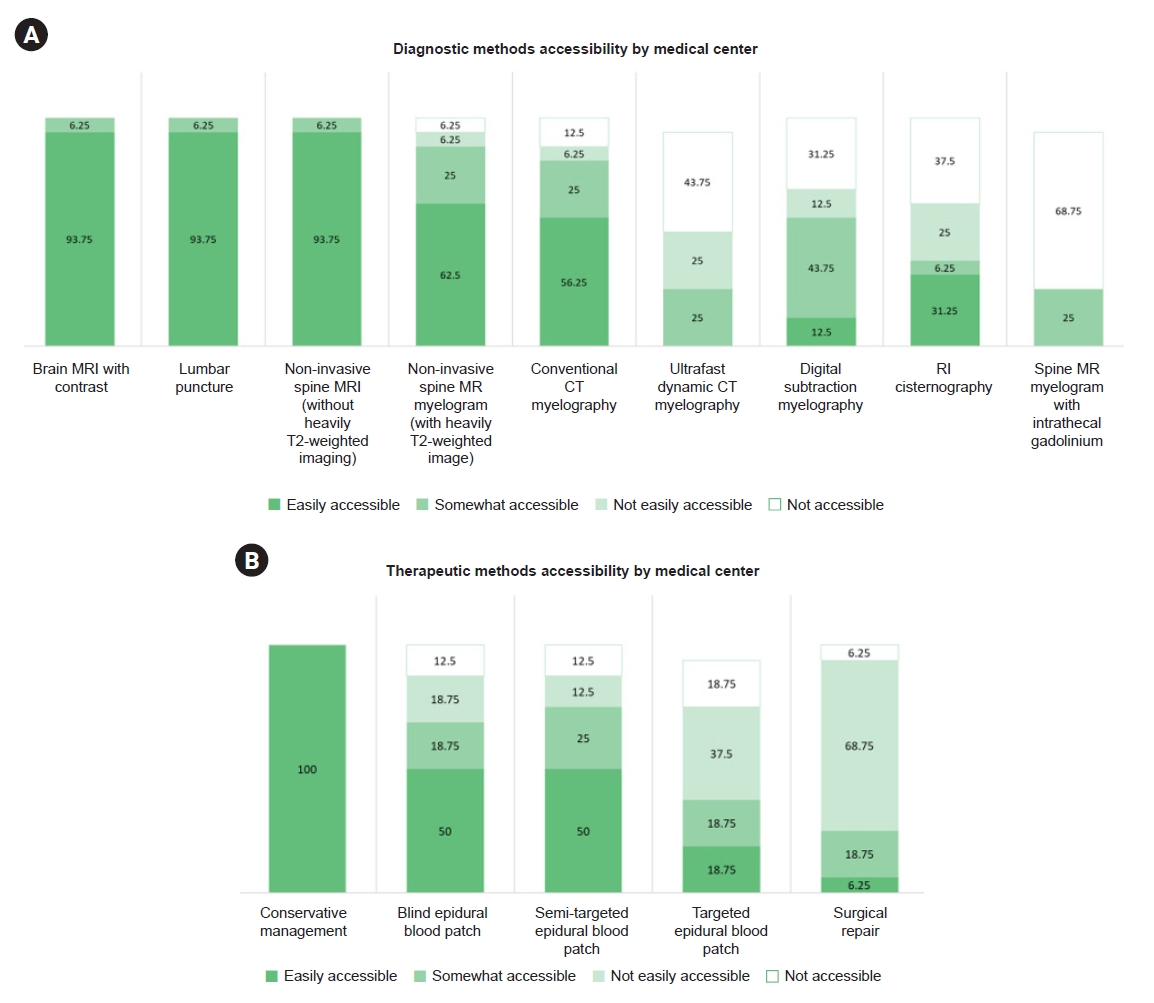
Accessibility of diagnostic and therapeutic methods by medical center. (A, B) It represent cumulative 100% bar graphs illustrating diagnostic and therapeutic accessibility based on responses by medical centers. The numbers above the bars indicate the percentage of each response.
MRI, magnetic resonance imaging; MR, magnetic resonance; CT, computed tomography; RI, radioisotope.
5. Usage of each diagnostic and therapeutic method at the individual level
At the individual practitioner level, 16 participants (80.00%) responded that they frequently used brain MRI, and noninvasive spinal MRI with or without heavily T2-weighted imaging was frequently used by 11 (55.00%) and 10 participants (50.00%), respectively (Figure 5A). Only eight (40.00%) and three patients (15.00%) frequently underwent lumbar puncture and conventional CT myelography, respectively, despite their easy accessibility, potentially suggesting their limited diagnostic value. No practitioners frequently used ultrafast CT myelography and only three (15.00%) used it occasionally. For treatment, only seven (35.00%) and five participants (25.00%) frequently used blind and semi-targeted EBP, respectively (Figure 5B). In contrast, none of the participants reported frequently using targeted EBPs or surgical repair.
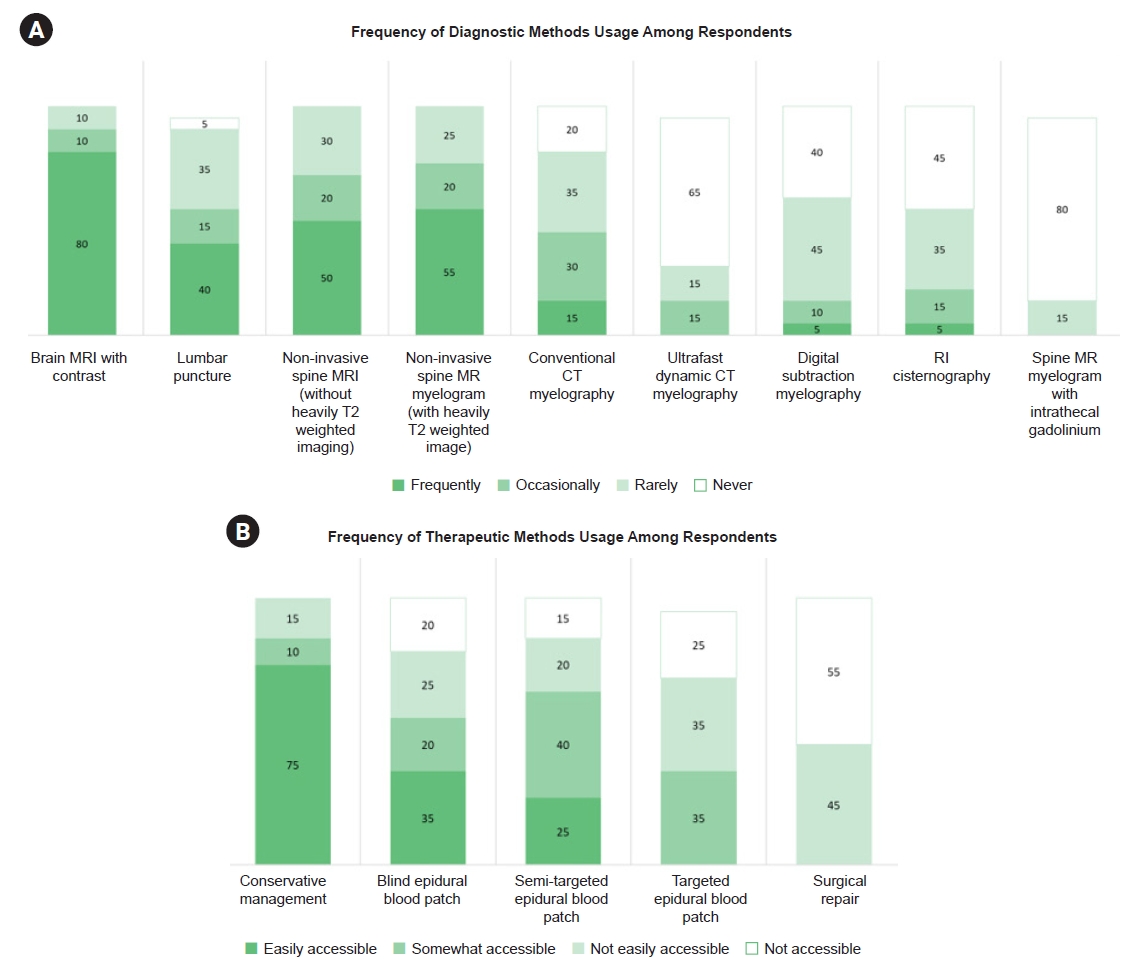
Usage of diagnostic and therapeutic methods by practitioner. (A, B) It represent cumulative 100% bar graphs illustrating diagnostic and therapeutic usage. The numbers above the bars reflect the percentage of each response.
MRI, magnetic resonance imaging; MR, magnetic resonance; CT, computed tomography; RI, radioisotope.
6. Barriers to using invasive techniques
Figure 6 summarizes the barriers to using advanced techniques, including ultrafast dynamic CT myelography, DSM, targeted EBP, and surgical repair. Physician unfamiliarity was the most common barrier in all four modalities. Notably, belief that the method is unnecessary, was a significant reason for the use of ultrafast CT myelography, DSM, and targeted EBP. Conversely, patient-related factors such as reluctance were more prominent during surgical repair. Cost and insurance limitations are rare barriers.
DISCUSSION
The significant findings of this study are as follows: 1) In most Asian countries, lumbar puncture, brain MRI, and spinal MRI are easily accessible, whereas real-time techniques, such as ultrafast CT myelography or DSM, are generally not easily accessible. 2) Although blind or semi-targeted EBP was available in most countries and centers, it was easily accessible in only half of the centers, and less than half of the participants used it frequently. 3) Surgical repair is not easily accessible in most centers and is never used frequently.
With an understanding of the imaging and clinical characteristics of SIH changes, methods for diagnosing SIH have also evolved. Previously, opening pressure was considered an essential component of diagnosis.11 However, understanding its pathophysiology has evolved from intracranial pressure to spinal CSF leakage. Consequently, lumbar puncture is no longer recommended for the diagnosis of SIH.12 To identify spinal CSF leakage, CT myelography has been considered as the diagnosis of choice. More recently, heavily T2-weighted noninvasive MR myelography was introduced as superior to conventional CT myelograms, offering a higher spatial resolution that enables sensitive identification of SLEC and lateral CSF leak without the risk of dural puncture.6 In our study, MR myelography was easily accessible in only one country at the national level despite the wide availability of spine MRIs. MR myelograms seem to be underutilized by our participants, who are headache experts or SIH investigators. MR is the safest and most sensitive method for identifying extradural fluid, so its use should be encouraged.
Ultrafast CT myelography and DSM enable the location of the exact site of a spinal CSF leak and planning treatment targeting the hole. Although locating the hole is crucial for surgical treatment, our study showed that these examinations are still not easily accessible in most Asian countries and centers and are not frequently used by Asian headache experts or SIH researchers. Interestingly, ultrafast CT myelography and DSM are available in most countries. However, because these examinations require multidisciplinary cooperation between different subspecialties, physician-related factors have been reported as the most compelling barriers.
Regarding treatment, this study showed the underutilization of EBP in Asian countries and centers. Blind or semi-targeted EBP was not easily accessible in approximately half of the centers despite its wide availability. Furthermore, fewer than half of the participants frequently performed blind or semi-targeted EBPs. This unique pattern may suggest challenges in performing invasive procedures in Asians. Given the low response rate to conventional “natural healing,” there is a need to improve both patient awareness and physician access to EBP. Multidisciplinary approaches for definitive targeted EBP and surgical treatment should also be encouraged.
Moreover, further discussion regarding EBP strategies appears warranted. As a result of limited access to ultrafast dynamic CT myelography or DSM, targeted EBP was not readily accessible in most centers across Asia. A recent study demonstrated that when EBP was performed targeting the periradicular leak level identified by MR myelography, the cumulative response rate reached 97.4% after up to three procedures.13 This suggests the potential for achieving favorable treatment outcomes even with noninvasive evaluation. Nevertheless, for patients who do not respond to EBP, targeted EBP or surgical treatment may be required. Therefore, appropriate patient selection for real-time imaging techniques is crucial, and multidisciplinary approaches should also be encouraged. Our findings emphasize the need for healthcare policymakers to address the disparities in access to advanced imaging and treatment of SIH. Reimbursement policies should be improved to ensure accessibility to such modalities. Establishing regional referral centers and training programs could enhance diagnosis and treatment quality. Further research should focus on outcomes associated with various diagnostic pathways and their cost-effectiveness in different healthcare systems.
This study had several limitations. First, the survey was conducted among a selected group of headache specialists from Asian countries. Although the respondents were experienced specialists, their perspectives and responses may not fully reflect the complete picture of each country’s SIH diagnosis and treatment practices, which may have resulted in a selection bias. Second, as the survey relied on self-reported data, it is subject to potential over- or underestimation of actual availability and usage. Differences in interpretation of survey questions may also have influenced the responses. Additionally, the survey responses were based on respondents selected from four subjective options, which may have introduced bias owing to individual linguistic differences. Third, several Asian countries were excluded from the survey. Despite conducting a systematic literature search to identify SIH experts in each country, there were some countries where information could not be obtained, and there were also instances where specialists did not respond to the survey. However, this study has several strengths. This is the first survey to involve investigators from different Asian countries to provide valuable insights into the current landscape of SIH diagnostics and therapeutics. We also used quantitative and qualitative methods to ensure the response accuracy.
Conclusion
In summary, this study highlights the variability and challenges in the availability, accessibility, and utilization of SIH diagnostic and treatment modalities in Asian countries. While almost all diagnostic methods are technically available, advanced imaging techniques for identifying CSF leaks remain less accessible, limiting the accessibility to surgical treatment options. The evolving understanding of SIH underscores the need for increased awareness and standardization of diagnostic methods among healthcare providers in Asia.
Notes
AVAILABILITY OF DATA AND MATERIAL
The data presented in this study are available upon reasonable request from the corresponding author.
AUTHOR CONTRIBUTIONS
Conceptualization: SJC, SKK, MJL; Data curation: SC; Formal analysis: SC; Investigation: SC, WSH, SKK, MJL; Methodology: SC, MJL; Project administration: SJC, MJL; Resources: SJC, AÖ, BB, EE, KV, KH, LTN, MS, MKC, OL, RI, SY, SPC, SJW, TT(Takeshima), TT(Takizawa), VKN, WTC, YFW, SKK, MJL; Supervision: MJL; Visualization: SC; Writing–original draft: SC, WSH; Writing–review & editing: SJC, AÖ, BB, EE, KV, KH, LTN, MS, MKC, OL, RI, SY, SPC, SJW, TT(Takeshima), TT(Takizawa), VKN, WTC, YFW, SKK, MJL.
CONFLICT OF INTEREST
Soo-Jin Cho is the Editor-in-Chief of Headache and Pain Research and was not involved in the review process of this article.
Mamoru Shibata is the International Editor of Headache and Pain Research and was not involved in the review process of this article.
Ryotaro Ishii is the Junior Editor of Headache and Pain Research and was not involved in the review process of this article.
Tsubasa Takizawa is the International Editor of Headache and Pain Research and was not involved in the review process of this article.
Soo-Kyoung Kim is the Deputy Editor of Headache and Pain Research and was not involved in the review process of this article.
All authors have no other conflicts of interest to declare.
FUNDING STATEMENT
Not applicable.
ACKNOWLEDGMENTS
We extend our sincere appreciation to the following individuals for their invaluable contributions to round 1 of our survey: Anan Srikiatkhachorn, MD, from Faculty of Medicine, King Mongkut’s Institute of Technology Ladkrabang, Bangkok, Thailand; Byung-Su Kim, MD, PhD, from the Department of Neurology at Ewha Womans University Mokdong Hospital, Ewha Womans University School of Medicine in Seoul, Republic of Korea; Charles Hua Chiang Siow, MD, of Mount Alvernia Hospital in Singapore; Danny Tat Ming Chan, MD, from the CUHK Otto Wong Brain Tumour Centre, Division of Neurosurgery, Department of Surgery at Prince of Wales Hospital, The Chinese University of Hong Kong in Hong Kong; Fumihiko Sakai, MD, PhD, from the Saitama International Headache Center at Saitama Neuropsychiatric Institute in Saitama, Japan; Jiying Zhou, MD, PhD, from the Department of Neurology at The First Affiliated Hospital of Chongqing Medical University in Chongqing, China; Julia Shahnaz Merican, MD, from the Neurology and Neurosurgery Centre at Princecourt Medical Centre in Kuala Lumpur, Malaysia; and Karthik Nagaraj, MD, from the Department of Neurology at Bangalore Medical College and Research Institute in Bengaluru, India.
SUPPLEMENTARY MATERIAL
Supplementary materials are available from https://doi.org/10.62087/hpr.2025.0005.
Search strategy
Selection process of survey participants by country of affiliation




