Advances in Primary Stabbing Headache: Diagnostic Criteria, Epidemiological Insights, and Tailored Treatment Approaches
Article information
Abstract
Primary stabbing headache (PSH), characterized by sudden, localized stabbing headache pain, is a recognized primary headache disorder with evolving diagnostic criteria. Epidemiological studies show a wide range of prevalence, influenced by various factors. PSH is more common in females, frequently occurring in conjunction with migraine, and can manifest in children. Recent diagnostic criteria have changed the definition of sharp stabbing pain, which is no longer restricted to the first division of the trigeminal nerve. In addition, the criterion of “no accompanying symptoms” has been refined to “no cranial autonomic symptoms” specifically. These changes have increased the sensitivity for capturing PSH. Although it is generally considered benign, stabbing headache can be associated with secondary causes. Clinical red flag signs can be helpful in distinguishing secondary headaches from PSH. A recent prospective study has proposed the monophasic, intermittent, and chronic patterns as subtypes, and this division may be helpful for predicting the prognosis. Pharmacological treatment is typically not required for PSH, although indomethacin and other alternating agents can be used. The treatment should be selected based on individual clinical features and comorbidities. This review aims to highlight the necessity of recognizing the distinctive clinical profile of PSH and of tailoring treatment approaches to patients’ individual needs.
INTRODUCTION
Primary stabbing headache (PSH), first described by Lansche1 in 1964 as “ophthalmodynia periodica,” is a well-recognized primary headache disorder. PSH is a sudden, localized stabbing pain in the head, known by various names such as ice-pick pain, jabs and jolts, needle-in-the-eye syndrome, and sharp short-lived headache. As different clinical features have been reported compared to those previously documented, the diagnostic criteria for PSH have changed over time. Initially, it was believed that the headache was confined to the first division of the trigeminal nerve.2 However, it has been reported that PSH can occur in extra-trigeminal areas, with PSH occurring in an extra-trigeminal area in approximately more than 70% of patients with PSH.3,4 Additionally, the criteria for no accompanying symptoms have been refined to specifically no cranial autonomic symptoms.5,6 The absence of cranial autonomic symptoms of PSH be helpful in differentiating it from short-lasting unilateral neuralgiform headache attacks with autonomic symptoms (SUNA) or short-lasting unilateral neuralgiform headache attacks with conjunctival injections and tearing (SUNCT). Consequently, PSH is now defined in the International Classification of Headache Disorders, third edition (ICHD-3), as a primary headache characterized by a single stab or a series of stabs that last for a few seconds, occurring with irregular frequency and without any cranial autonomic symptoms.6 Although the pathophysiology of PSH is unknown, current hypotheses include irritation of the trigeminal and extra-trigeminal nerves and/or intermittent impairment of central pain processing leading to neuronal hyperexcitability or spontaneous synchronous discharge of neurons. Previous studies have shown that most cases of stabbing headache are benign and self-limiting without treatment. However, a few studies have reported structural intracranial or extracranial disorders and systemic autoimmune disorders,7,8 suggesting that neuroimaging may be necessary when clinical features do not match the typical features of PSH. With regard to treatment, PSH is regarded as one of the indomethacin-responsive headaches.9 As indomethacin can demonstrate inadequate response and may contraindicate or be intolerable to individual patients, a number of different treatments have been attempted, with some demonstrating efficacy in clinical trials despite small case studies. Treatment may therefore be selected based on individual clinical features and comorbidities. This review aims to provide an up-to-date review of epidemiology, clinical features, diagnostic criteria, differential diagnosis, and treatment of PSH.
EPIDEMIOLOGY
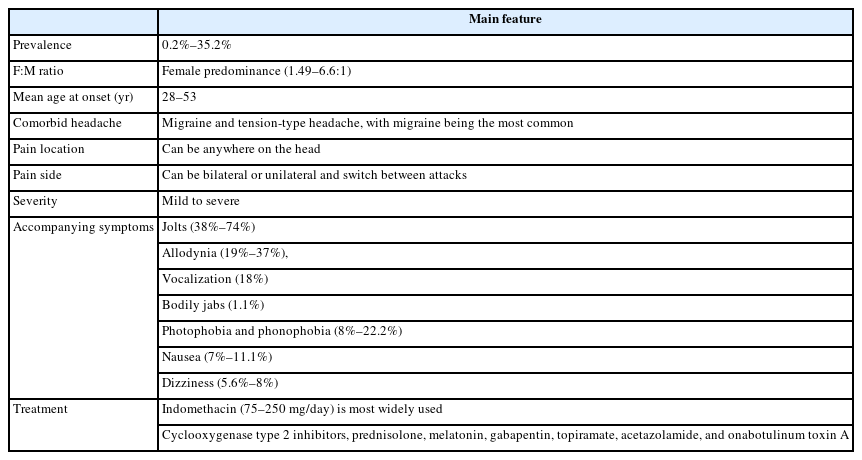
The prevalence of PSH exhibit significant variability across different epidemiological studies. This variability is influenced by several factors, including age, sex, referral bias, the definition of PSH, and the presence of comorbid headache disorders. Reported prevalence rates for PSH range from 0.2% to 35.2% in general population and 1.5% to 26.7% in hospital- and clinic-based studies, respectively. In general population, the largest study specifically examining PSH prevalence was conducted in Vågå, Norway, where 1,779 parishioners were questioned about head pain described as jabs.10 This study reported a high lifetime prevalence of 35.2%. In contrast, other studies have reported significantly lower prevalence. In a large study of primary headache disorders, 1,000 individuals in Copenhagen were interviewed, and only 2% reported a lifetime occurrence of stabbing headache.11 Similarly, a population study in Porto involving 2,008 subjects found a lifetime prevalence of only 0.2%.12 In addition, hospital- and clinic-based studies have been performed to investigate the prevalence. A study of 1,219 patients presenting to a tertiary neurology clinic in China found that isolated PSH had a prevalence of 1.5%.13 In a Turkish headache clinic, a prevalence of 12.6% were reported.14 In Spanish and Taiwanese headache clinics, 5% of 725 patients and 13% of 872 patients, respectively, were reported.15,16 In Korea, the self-reported lifetime prevalence rates of PSH were 11.0% among patients with headache and 26.7% among neurologists in different hospitals.17,18 These differences between patients and neurologists might reflect under diagnosis of PSH in general populations.
PSH is more common in females within the adult population, with a female-to-male ratio ranging from 1.49 to 6.6:1.10,19 The mean age of onset for PSH in adults ranges from 28 to 53, with significant variation across previous studies.10,16,20 A family history of migraine was reported in 34.8%–40.5% of patients with PSH.21,22 PSH can occur in isolation or in association with other headache types, including migraine and tension-type headache, with migraine being the most common. A prevalence of approximately 40% has been reported in patients with migraine.23
In childhood, the prevalence of PSH ranges from 3.35% to 9.97%.24-26 Age of onset for PSH is between 4.5 and 9 years, with 12.4% of cases occurring in children younger than 6 years old.27 Recently, the prevalence of PSH were found to be 77 patients (9.97%) of 772 children and adolescents, indicating that it is not uncommon among thisagegroup.26 In this study, 0.9% of patients experienced the onset of PSH before the age of six, with a mean onset age of 10.9±3.5 years (ranging from 4 to 16 years).26 The sex distribution among children with PSH is not consistent across studies.
CLINICAL CHARACTERISTICS
Patients typically describe the pain as stabbing or piercing without a pulsatile component.28 This stabbing pain occurs spontaneously and in irregular patterns, with no circadian or circannual rhythm, and can even wake patients from sleep.16 The severity of pain reported by patients shows considerable variation across different studies. While the Vågå study found that 93% of patients described their pain as mild to moderate, other studies indicate that the majority of patients experience pain that is moderate to severe.20 Pediatric studies show no significant difference in pain severity, with most patients reporting moderate to severe pain. Based on the previous epidemiological and clinical studies, 80% of attacks last 3 seconds or less, although occasionally they can last from 10 to 120 seconds.16,19 The majority of patients experience single, brief stabs of pain, although they may also present with a series of stabs. Attacks of stabbing pain are typically infrequent, occurring one to a few times a day. However, on rare occasions, the pain can occur repeatedly for days to 1 week.6,16,19 At times, patients may experience more than a dozen attacks per day. Initially, the pain was believed to be localized to the first branch of the trigeminal nerve, with previous studies indicating that 45%–62% of patients with PSH experienced pain exclusively in the V1 distribution.16,19 However, more recent research indicates that up to 70% of patients also experience stabbing pain in areas other than the trigeminal nerve, including the occipital, nuchal, and parietal regions innervated by nerves C2–C4.16 The stabbing pain may manifest unilaterally or bilaterally, with unilateral location reported in 59%–91.4% of patients.3,29 During the stabs, symptoms such as jolts, allodynia, vocalization, and bodily jabs can be accompanied. Among them, jolts and allodynia are commonly observed. Jolts can accompany the stabbing pain in 38%–74% of cases, while allodynia is present in 19%–37% of patients with PSH.4,16 Vocalization was observed in 18% of patients, while bodily jabs were seen in only 1.1% of those with PSH.16,30 Nausea and vomiting (7%–11.1%), photophobia and phonophobia (8%–22.2%), and dizziness (5.6%–8%) were uncommon accompanying symptoms.13,16,24 In contrast to trigeminal autonomic cephalalgias (TACs), PSH do not present with cranial autonomic features such as tearing or ptosis during the pain attacks. Although similar findings have been observed in pediatric studies, one study reported that vertigo, nausea, photophobia, and phonophobia can occur in as high as 47% of children with stabbing headache.24
In recent studies, clinical courses and patterns of PSH have been proposed, including monophasic, intermittent, and chronic.4,31 The patterns were identified based on the frequency of stabbing pain, the clinical course, and the total disease duration. Stabbing pain in the monophasic pattern was characterized by greater severity, higher frequency, side-locked location, and single stabs that typically responded well to treatments such as indomethacin, steroids, gabapentin, or tricyclic antidepressants. In contrast, the chronic daily stabbing pain pattern was associated with a longer duration, variable location, multiple stabs, less responsiveness to treatment, and a higher prevalence among female patients.4
DIAGNOSTIC CRITERIA
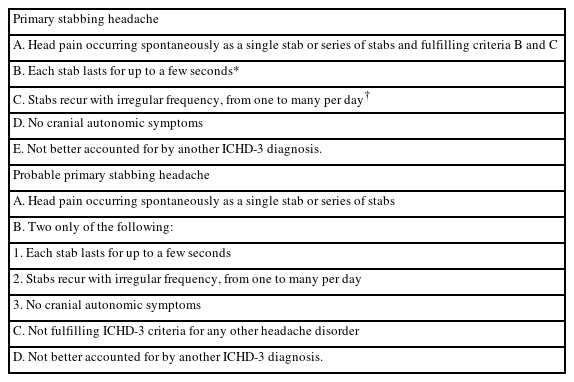
The diagnosis of PSH is based on the clinical characteristics outlined in the ICHD criteria (Table 1).6 Over time, there have been two significant changes to the diagnostic criteria for PSH. The first is the location of pain, and the second is the accompanying symptoms. In the ICHD-2 criteria published in 2004, the diagnosis of PSH was restricted to the first branch of the trigeminal nerve.5 However, according to some later studies, PSH was localized in extra-trigeminal regions such as behind the ear, frontal, parietal, and occipital regions, implying that the head can be all involved. Consequently, in 2018, the ICHD-3 criteria, which limited the location of pain to the first branch of the trigeminal nerve, were abandoned.6 In accompanying symptoms of ICHD-2, no accompanying symptoms were included for the diagnosis of PSH. However, accompanying symptoms such as allodynia, nausea and vomiting, and photophobia or phonophobia have been reported. Consequently, the ICHD-3 revised the diagnostic criteria for PSH, changing no accompanying symptom to no cranial autonomic symptoms.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis for PSH encompasses a range of short-lasting, stabbing primary and secondary headache disorders. Among the primary headache disorders, the short-duration primary headache category includes trigeminal neuralgia, TACs such as paroxysmal hemicrania, SUNA and SUNCT. Trigeminal neuralgia is a unilateral disorder characterized by brief electric shock-like attacks of pain limited to the distribution of one or more divisions of the trigeminal nerve. The attacks of pain are often precipitated by mechanical stimulation such as speaking, eating, or brushing teeth. The distribution of pain and provocative factors differentiate trigeminal neuralgia from PSH. TACs must have unilateral cranial autonomic features by definition. The presence or absence of autonomic symptoms represents a key differentiating factor between a TAC and PSH.
Stabbing headaches have been described as the presenting symptom in pituitary tumours,32 intracranial meningioma,33 herpetic meningoencephalitis,34 stroke,35 and giant cell arteritis.36 Therefore, underlying pathology should be considered in patients presenting with new complaints of stabbing headaches. Furthermore, there have been reports of stabbing headaches in patients with autoimmune disorders, including multiple sclerosis, lupus, Behcet’s disease, Sjogren’s syndrome, vasculitis, antiphospholipid antibody syndrome, and Lyme disease.8 A recent studies have identified clinical red flag signs that are associated with secondary causes. These include the recent onset of stabbing headache, which is exclusively unilateral (ipsilateral) at the same location, a crescendo pattern, which is triggered by head movements, or a Valsalva maneuver.7 Although the prevalence of secondary headache disorders manifesting as stabbing headaches was low, brain imaging such as computed tomography or magnetic resonance imaging could be considered to investigate for potential secondary structural disorders. It is also reasonable to perform blood evaluation, including erythrocyte sedimentation rate, in patients over the age of 50 who present with stabbing pain, particularly if they have additional features of giant cell arteritis.
PATHOPHYSIOLOGY
The pathophysiology of PSH remains unclear. Proposed theories include irritation of peripheral branches of the trigeminal nerve or other cranial nerves, as well as intermittent dysfunction of central pain processing leading to spontaneous synchronous discharges or hyperexcitability of neurons.37,38 It is hypothesized that irritation or spontaneous firing of the peripheral branches of the trigeminal nerve cause stabbing pain. The pain of PSH, which is very short-lasting, focal, and not triggered by external stimuli, suggests a spontaneous and temporary firing of nociceptive nerve endings, specifically A-delta fibers, originating from sensory afferents in the head, such as the trigeminal and occipital nerves.16 The intermittent nature of most PSH, characterized by infrequent stabs and normal sensory function between episodes, implies a nonpathological process without axonal damage. Nociceptive Schwann cells, recently identified in both mice and humans, might fail transiently in their pain gating function, leading to spontaneous nerve firing.39 However, as the location of pain often does not correspond to the distribution of the trigeminal nerve, the pathogenesis is likely to be more complex.37
Dysfunction of central pain processing secondary to peripheral mechanisms can be another proposed mechanism. Extra-trigeminal stabbing headaches can occur in cephalic regions innervated by C2–C4. Pain signals from these upper cervical areas, similar to those from trigeminal inputs, are transmitted to the trigemino-cervical complex. This suggests that both trigeminal and extra-trigeminal sources may converge on central pain control mechanisms that may be defective.16 Central sensitization may be another central mechanism for intermittent and chronic daily PSH. This may explain the long-term course, migrating pain locations, allodynia, and bodily jabs observed in PSH patients. High prevalence of PSH with migraine has also suggested the proposed segmental disinhibition of central pain processing.38
TREATMENT
In patients with infrequent attacks, the explanation that PSH is a benign condition may be sufficient and treatment may not be necessary. However, high frequency of stabbing attacks may require intervention. Due to pain paroxysms, treatment is aimed at prophylactic suppression of the attacks. PSH is considered to be one of the indomethacin-responsive headache syndromes.40 In PSH, the therapeutic mechanism of indomethacin may be its anti-inflammatory and vasoconstrictive properties.41,42 It is a nonsteroidal anti-inflammatory drug that exerts its effects through reversible inhibition of cyclooxygenase (COX)-1 and COX-2 enzymes. It also impedes polymorphonuclear leukocyte motility, reduces mucopolysaccharide synthesis, and may induce vasoconstriction.42 Furthermore, indomethacin is known to inhibit nitric oxide release, decrease cerebral blood flow, and lower cerebrospinal fluid pressure.41 Patients with PSH have been reported to respond quickly to indomethacin ranging from 75–250 mg/day given in divided doses. Dose-related side effects include dyspepsia, gastrointestinal bleed, and renal toxin.40 Long-term treatment with indomethacin may also result in gastrointestinal and renal side effects. Furthermore, the response rate to indomethacin was found to be inconsistent across clinical studies, with remission rates ranging from 20% to 57%.15,16,19 Therefore, other treatment options for patients who are unable to tolerate indomethacin or are not responsive to indomethacin.
Alternative agents reported to be effective in small series of patients include other COX-2 inhibitors,43-45 prednisolone, melatonin,46 gabapentin,47 topiramate,48 acetazolamide,49 amitriptyline,50 and onabotulinum toxinA.51 In cases where a series of treatments utilizing selective COX-2 inhibitors such as celecoxib and etoricoxib were used, a favourable response was observed. Melatonin is a pineal hormone and marker of circadian function. Its chemical structure is very similar to that of indomethacin. In cases series, patients had complete remission with melatonin, 3–21 mg at night.46 In this study, a strategy which starting with a bedtime dose of 3 mg and increasing by 3 mg every three to four nights until pain relief, with 24 mg as an upper dose limit was recommended. In other cases, gabapentin 400 mg every 12 hours have also been reported to be effective.47 All patients had complete relief of pain already in the first days of treatment. The precise mechanisms of action of gabapentin are still not completely defined but are probably related to both peripheral and central pathways of pain suppression. Gabapentin may have an effect by inhibiting ectopic discharge activity from injured peripheral nerves.52 In case reports, topiramate 100 mg per day and acetazolamide 250 mg twice per day was effective in the treatment of PSH.48,49 In other case series, two patients were treated with amitriptyline, a single dose of 10–25 mg at bedtime with significant improvement within the first month of treatment.50 Onabotulinum toxinA was studied in a prospective, unblinded study in 24 patients with PSH.51 Patients received five units of onabotulinum toxinA into each area where they experienced the stabs with the mean dose 11.81±7.17 units. Among them, 22 patients showed partial-response and three patients full-response.
The treatment plan for PSH could be tailored according to the specific clinical pattern presented in a recent prospective study: monophasic, intermittent, and chronic.4 For a monophasic pattern, characterized by a single episode, short-term treatment with indomethacin, COX-2 inhibitors and/or prednisolone can provide quick relief. For an intermittent pattern, involving sporadic episodes over weeks to months, preventive and abortive treatments with indomethacin and/or melatonin, gabapentin, topiramate, acetazolamide, or amitriptyline may reduce frequency and severity. Chronic patterns, defined by daily or near-daily episodes, require consistent preventive approaches with long-term use of indomethacin and/or botulinum toxinA injections, along with regular monitoring and dosage adjustments. Adjunctive therapies such as lifestyle modifications, including regular sleep patterns, stress management, and avoiding known triggers, can further help manage headache frequency and severity.
While there is limited information available regarding the treatment of PSH in children and adolescents, it’s noted that the number of reported cases is small. Nonetheless, the general treatment strategy should be similar to that of adult patients.
PROGNOSIS
Previous studies of PSH have indicated that both population-based and clinic-based subjects exhibited a wide variety of disease durations, ranging from a few days to several years.10,16 Although there have been no prospective studies on the prognosis of PSH, it is generally regarded as a benign condition that may resolve over time. However, in a recent study, half of patients with PSH experienced spontaneous remission, while the other half required medical treatment to reach remission. This study has indicated that the prognosis of PSH may depend on the individual clinical courses.4 Patients with the intermittent subtype may experience relapses at variable frequencies over several years. This subtype may progress to the chronic daily subtype, although this has been reported in a very small number of patients. In patients with the chronic subtype, the majority may not respond to treatment and continue to experience chronic daily headaches. The chronic daily pattern was associated with multiple or migrating locations, slightly longer-lasting stabs, frequent allodynia, and bodily jabs.4
SUMMARY
Clinical features of PSH were summarized on Table 2. The evolution of diagnostic criteria has expanded understanding of its clinical features and epidemiology, revealing a wide prevalence range influenced by demographic factors. While treatment options such as indomethacin and alternative agents exist, prognosis varies among patients. Further research is needed to elucidate the underlying pathophysiology and optimize management strategies for PSH. This comprehensive review serves to highlight the necessity of recognizing the distinctive clinical profile of PSH and of tailoring treatment approaches to the individual needs of patients.
Notes
AVAILABILITY OF DATA AND MATERIAL
Not applicable.
AUTHOR CONTRIBUTIONS
Conceptualization: SC; Data curation: SC; Investigation: SC; Writing–original draft: AC (Ayush), SC; Writing–review and editing: AC (Avinash), SC.
CONFLICT OF INTEREST
Soohyun Cho is the Editor of Headache and Pain Research and was not involved in the review process of this article. All authors have no other conflicts of interest to declare.
FUNDING STATEMENT
Not applicable.
ACKNOWLEDGMENTS
Not applicable.