Premonitory Symptoms in Migraine: Implications for Disease Burden and Cognitive Impairment, with Some Promising Answers
Article information
Abstract
Purpose
This study evaluated the prevalence and impact of premonitory symptoms (PS) in people with migraine, assessing their influence on disability, cognitive function, and quality of life.
Methods
In a cross-sectional analysis at Mersin University Hospital, 186 migraine patients were interviewed to identify the presence of PS, using a structured questionnaire that included measures of disability (Migraine Disability Assessment Scale or MIDAS), quality of life (European Health Impact Scale or EUROHIS-8), and cognition (Migraine Attack Related Subjective Cognitive Scale or Mig-SCOG). Statistical analyses included descriptive statistics, the t-test, and the Mann-Whitney U-test, with a significance threshold set at p<0.05.
Results
Among participants, 74.7% reported one or more PS, with the most common being neck stiffness (64.7%), photophobia (56.8%), fatigue (52.8%), and phonophobia (50.3%). Patients with PS demonstrated significantly lower quality of life scores (EUROHIS-8, p<0.001) and higher cognitive impairment scores (Mig-SCOG, p<0.001) than those without PS, despite similar levels of migraine disability (MIDAS, p=0.050).
Conclusion
The high prevalence of PS in people with migraine and their association with greater cognitive impairment and reduced quality of life indicate that more targeted interventions are necessary in this subgroup. PS may be either a driver of cognitive and quality of life burden or just a marker of it, and disambiguating these possibilities will be a critical area for future research and clinical focus. More optimized and standardized prospective studies are needed to clarify the prevalence of PS.
INTRODUCTION
Migraine is a primary headache syndrome characterized by attacks of headaches preceded and followed by certain symptoms under appropriate exogenous and endogenous conditions. According to the Global Burden of a Disease 2019 Study,1 migraine is the second most common cause of disability worldwide after stroke. Patients with migraines have symptoms that predict the onset of a headache, and these symptoms are called premonitory symptoms (PS). These symptoms must be warning symptoms before the migraine headache occurs. In one study,2 symptoms that started up to 48 hours before a headache attack had a 72% probability of predicting a migraine attack. According to the International Classification of Headache Disorders (ICHD)-3,3 PS are defined as symptoms that may start up to 48 hours before the headache attack and are seen before the headache or aura. In the literature, 96 PS have been defined.4 These symptoms include fatigue, irritability, hunger, frequent urination, yawning, impaired concentration, memory problems, and mood changes.5 The prevalence of these symptoms varies between 7.8%–67.4%6-8 in population-based studies and 21.5%–100%2,9-12 in clinic-based studies. It is difficult to determine the exact prevalence of PS. The type of study, methodological differences, and the intertwining of symptoms with triggers, aura symptoms, and symptoms accompanying headache are among the difficulties encountered in determining prevalence. In a study conducted to determine PS with functional imaging,13 it was determined that regions such as the hypothalamus, dorsal pons, periaqueductal gray matter, and locus coeruleus were activated up to 24 hours before the headache attack and that the activation of these regions continued throughout the headache (Figure 1). For this reason, he argued that PS should be included in the migraine attack. It is also known that these regions are in the pain matrix and cause pain formation, loss of habitation, and pain behavior in migraine.14 Common anatomical structures are involved in the formation of PS as well as the regulation of pain habituation, pain behavior, and cognitive function. Although it is not known precisely how these structures function, several processes that activate these structures during a migraine attack may also affect cognition and pain. For this reason, patients who experience PS may have differences in pain frequency, intensity, duration, and cognitive impairment compared to those who do not. Therefore, their quality of life may have a more significant negative impact. Thus, we aimed to determine the differences between patients with and without PS, especially in disease burden and pain characteristics.
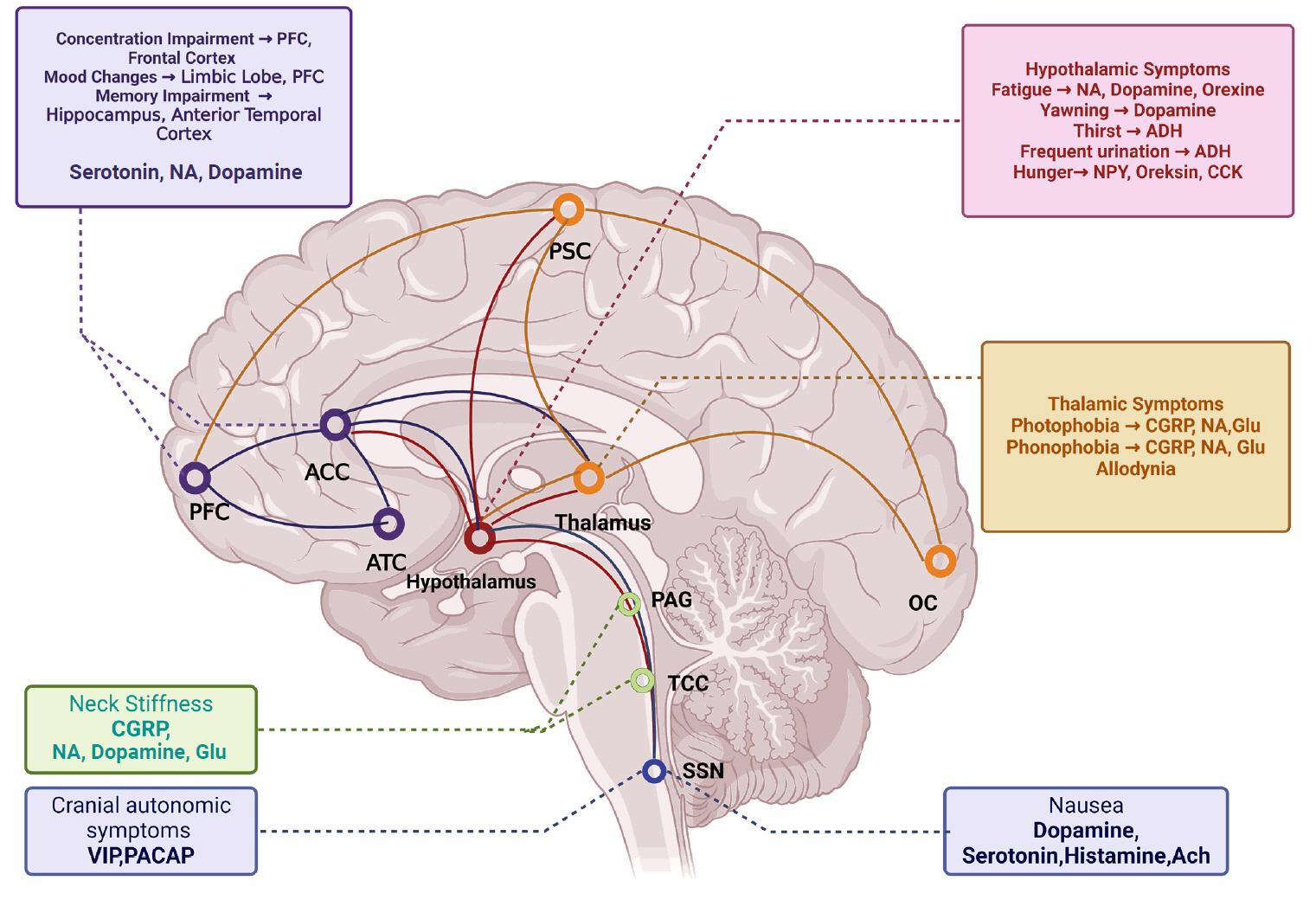
Hypothesized mechanisms of premonitory symptoms.
PSC, primary somatosensory cortex; PFC, prefrontal cortex; ACC, anterior cingulate cortex; ATC, anterior temporal cortex; OC, occipital cortex; PAG, periaqueductal gray matter; TCC, trigeminocervical cortex; SSN, superior salivatory nucleus; NA, noradrenaline; ADH, antidiuretic hormone; NPY, neuropeptide Y; CCK, cholecystokinin; CGRP, calcitonin gene-related peptide; Glu, glutamate; VIP, vasoactive intestinal peptide; PACAP, pituitary adenylate cyclase-activated peptide; Ach, acetylcholine.
The primary objective of this study is to establish the prevalence of PS in migraine patients at a tertiary headache center. Our secondary objective explores the frequency and types of PS experienced by these patients, as well as their contribution to migraine attacks and cognitive functions. The third objective is to compare the quality of life, cognitive functions, and disease-related disability between patients with and without PS, utilizing objective measurement scales.
MATERIALS AND METHODS
1. Study design and setting
This cross-sectional study was conducted at Mersin University Hospital, adhering to the principles of the Declaration of Helsinki.15 Approval was obtained from the Mersin University Clinical Research and Ethics Committee (approval No. E-78017789-050.01.04-1740527).
2. Participants
We included a total of 186 patients aged 18–70 years who presented with migraine with or without aura, who applied to Mersin University Hospital between September 2021 and October 2022, and met the diagnosis of migraine with aura and migraine without aura according to ICHD-3.3 We informed all the patients and obtained their consent before conducting the procedure. Exclusion criteria were cluster headache, hemiplegic migraine according to ICHD-3;3 suspected migraine diagnosis, medication-overuse headache, and tension-type headache added to migraine.
3. Sample size
Power analysis determined that a sample of 200 participants was required to achieve 80% power, considering the expected prevalence and effect size detected in PS.11,16
4. Data collection
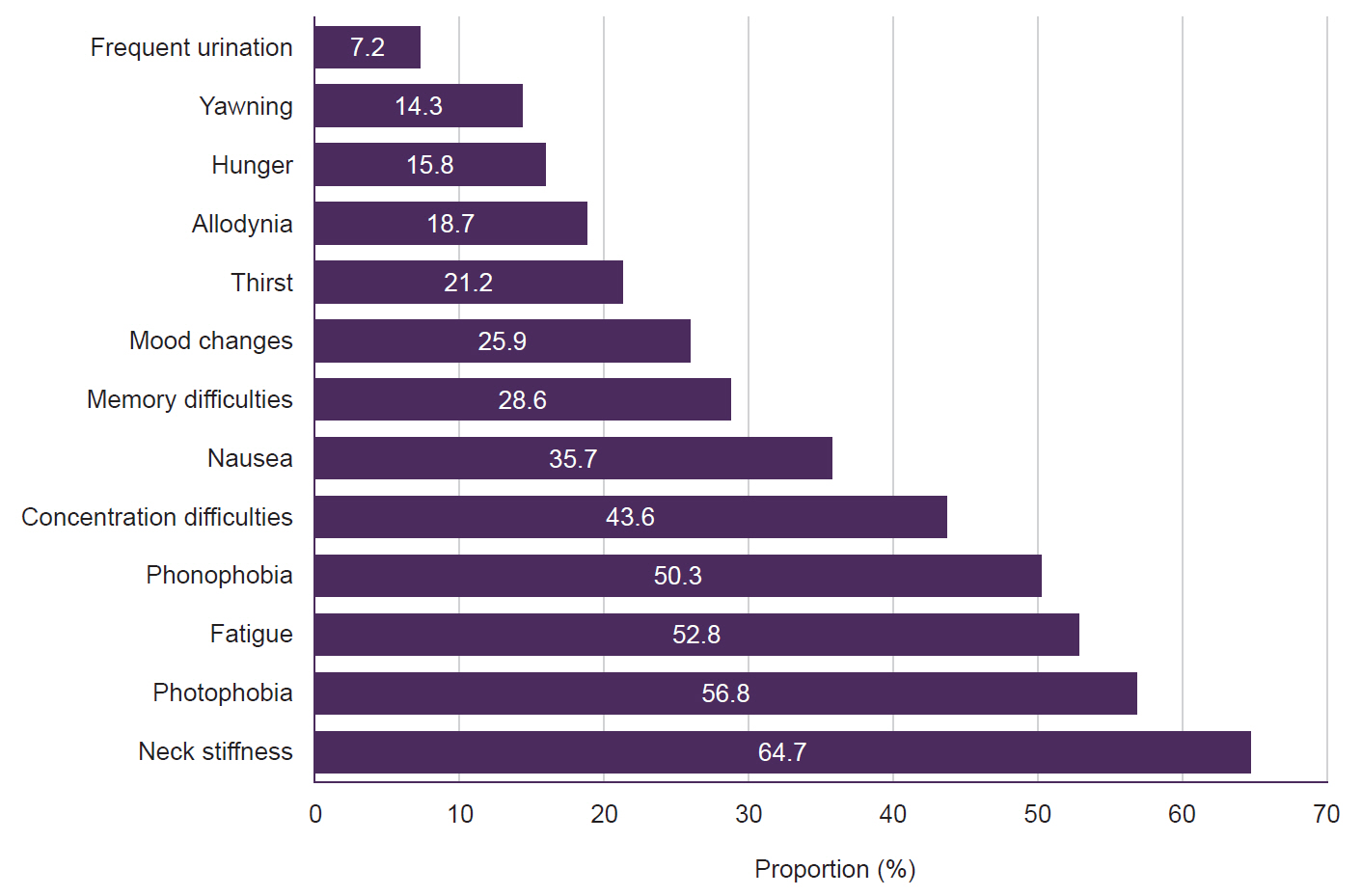
Eligible participants underwent a face-to-face structured interview that collected demographic data, migraine characteristics (type, number of headache days per month, pain intensity), and additional health information, including medication overuse and other headache types. The definition of migraine premonitory were made according to ICHD-3,3 with participants indicating the onset and frequency of these symptoms relative to their migraine attacks. For migraine patients with aura, complaints that start before the aura, and for migraine without aura, symptoms that occur before the onset of a headache attack are called PS. Our study did not accept the lower limit of 2 hours before the onset of pain in ICHD-217 and ICHD-3β18 criteria. We identified 13 PS (fatigue, yawning, photophobia, phonophobia, thirst, neck stiffness, concentration difficulties, memory difficulties, nausea, mood changes, allodynia, hunger, frequent urination) can be seen in Figure 2. While identifying the PS, we focused on those commonly seen in previous studies.19 The top five frequently seen symptoms were labeled as core PS, and remain eight symptoms were labeled as other PS in terms of symptom frequencies based on a multicenter Chronic Migraine Epidemiology and Outcomes20 study.
5. Procedure
Patients who met the inclusion and exclusion criteria and gave consent were examined to clarify whether they had migraines, and then asked questions in a face-to-face interview. The questions gathered demographic data about the patients and included inquiries regarding migraines, specifically the monthly migraine days (MMD), which denotes the number of headache days within a month, a visual analogue scale (VAS) to assess pain intensity, and the duration of migraine attacks. We questioned whether there was overuse of medication in addition to migraine, whether there was tension-type headache, whether they used prophylactic treatment and interventional treatment for migraine. Participants were accepted to the study after this preliminary interview (Figure 3).
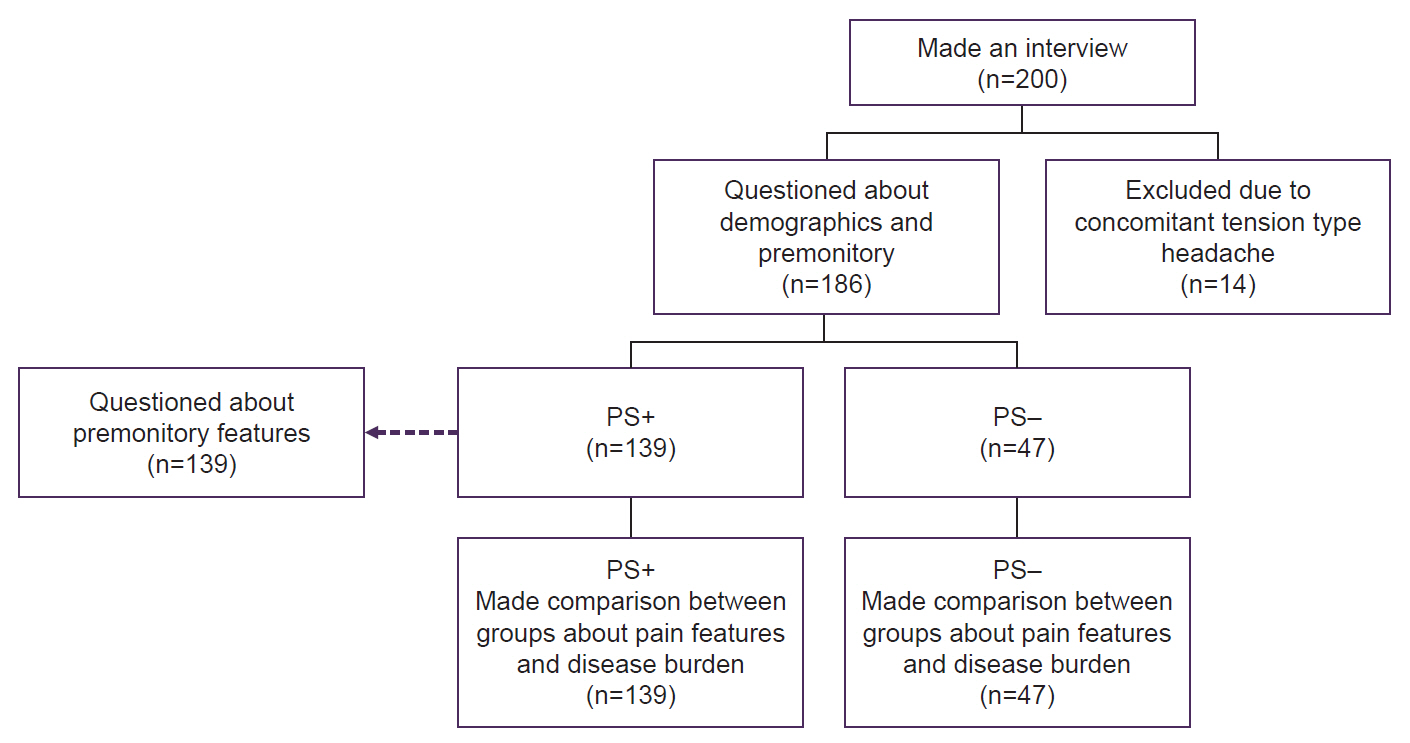
The study design (flow chart).
PS+, premonitory symptom positive; PS–, premonitory symptom negative.
We asked the participants whether they felt any warning symptoms before the headache. If the participants stated at least one or more PS positively, we counted them as PS positive (PS+). Then, we asked PS+ which of the 13 PS we identified from the list. We asked them how long before the pain the PS started and how many of these PS they experienced for every five headaches. We asked respondents who experienced PS 1–4 out of five headache attacks to compare headaches with PS to those without.
Afterward, we applied three different scales for disability and quality of life to patients with and without PS: Migraine Disability Assessment (MIDAS),21 European Health Impact Scale (EUROHIS-8),22 a shortened version of the World Health Organization Quality of Life scale, and Migraine Attack Related Subjective Cognitive Scale (Mig-SCOG).23 We used the MIDAS scores in our study; it as the sum of days lost due to headache in the last three months, and we treated it as a continuous score. The EUROHIS-8 scale is a test used to determine quality of life, there are 8 questions and each question is scored between 1–5. Scores range from 8–40, with lower scores indicating a decrease in quality of life. The Mig-SCOG is a subjective scale used to determine cognitive impairment during migraine headaches. It includes nine questions evaluating different cognitive domains. Scale scores range from 0 to 18. Higher scores indicate more pronounced cognitive impairment.
6. Statistical analysis
We performed the statistical analysis of the study in 3 stages. After processing the demographic data of the patients into the statistical program ‘TIBCO STATISTICA ver. 13.5.0’ (TIBCO Software Inc.) in the first stage, we determined the PS rate over the whole patient group with descriptive statistical analysis.24 In the second stage, for the questions directed to the patients with PS, we selected only the patient group with PS and determined the PS rates, when PS started, and how often they occurred. We performed network analyses using Jeffreys’s Amazing Statistics Programme (JASP) software.25 JASP is an open-source, downloadable statistical software program that conducts various statistical analyses. The network analysis served as a modeling and visualization strategy to illustrate the associations among multiple variables. This approach employs a multivariate graphical technique to model the interrelationships between numerous variables. Networks were composed of nodes and edges. Moreover, the color of edges represents either positivity (blue) or negativity (red) associations. The edge weights show the strength of the associations between nodes in the network. In addition, link analysis as an association rule mining was applied by using TIBCO STATISTICA v.13.5.0 (TIBCO Software Inc.) to generate rules based on probability calculations of the co-occurrence frequencies of PS and pain properties. Link analysis has the unique capability of handling continuous variables as well as categorical variables in a single analysis.24 The strength of association rules were determined based on the lift values. A lift ratio greater than 1 indicates a positive relationship between two variables. The lift value is an important measure in association analysis as it helps determine how meaningful the association rules are and how powerful they reflect real relationships. It is one of the most important criteria for selecting and interpreting association rules. The others are support and confidence values. Minimum support and confidence values were assumed as 20% and 10%.
In the third stage, we divided the patients with and without PS into two separate groups as PS+ and PS negative (PS–) (PS+: n=139, PS–: n=47). Then, we compared the pain severity, the number of headache days per month, and the results of the scales between these two groups. We used Kolmogorov-Smirnov analysis for normality analysis and used a t-test to compare numerical data with normal distribution and the Mann-Whitney U-test to compare numerical data that was not normally distributed. We used p-value to determine significance values and we considered p<0.05 as statistically significant.
RESULTS
1. Premonitory symptoms rate
Of the total 186 participants, 159 (85.5%) were females and 27 (14.5%) were males. The mean age of the participants was 35.54±10.35 years. One hundred and 48 (79.6%) of participants were episodic migraine and 38 (20.4%) was the chronic migraine. One hundred thirty-nine (74.7%) of the participants had PS.
2. Premonitory symptom analysis
Participants with PS included 117 female and 22 male. Among the 13 PS in our dataset, the four symptoms with the highest rates were neck stiffness (64.7%), photophobia (56.8%), fatigue (52.8%), and phonophobia (50.3%) (Figure 2).
PS typically begin 1–6 hours before the onset of head pain (72.6%). In all but one patient, symptoms started within 48 hours of headache onset. In about one in three participants PS occur with every attack. In three or more out of attacks, PS occur in 72.5% of patients. Of the PS+ participants, nearly half of patients (43.1%) with PS+ headaches reported that the PS+ headaches were worse, 16.7% of them stated headaches were same and 8.3% of them stated headaches were better. Rest 31.9% were unable to compare headache severity due to having PS all of their headache. Almost one-third (33.1%) of the PS+ cases reported almost all headache attacks started with PS.
The weights from weighted matrix among PS and pain properties were illustrated as Networks in Figure 4. The weights among PS, MMD, VAS, and headache duration revealed that, the strongest positive relationship (w: 1.345) found between MMD and frequent urination and negative association between MMD and hunger (w: –0.699) (Figure 4A). Headache duration was positively related to memory difficulties and dizziness (w: 1.081, w: 0.617, respectively) and inversely related to photophobia and concentration difficulties (w: –1.054, w: –0.545, respectively) (Figure 4B). For the VAS, there were positive relationship with dizziness, yawning, and frequent urination (w: 1.444, w: 1.178, w: 0.789, respectively), negative association with photophobia (w: –0.807).
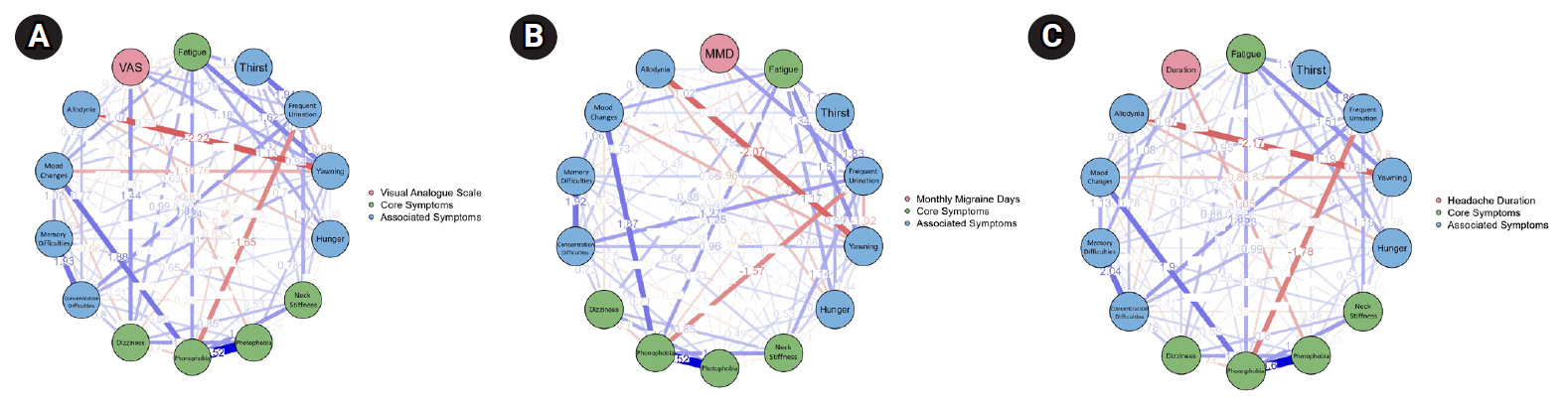
Association between pain properties (duration, monthly migraine days [MMD] frequency, and visual analogue scale [VAS] pain intensity) and premonitory symptoms in a weighted matrix (n=139). A weighted matrix between premonitory symptoms and pain properties is given in a schematic diagram. (A) The association between VAS and premonitory symptoms. (B) The association between MMD and premonitory symptoms. (C) The association between pain duration and premonitory symptoms. Core premonitory symptoms are marked in green, associated premonitory symptoms are marked with blue circles. Positive links are marked as blue lines, negative links are marked as red lines. Thicker lines show stronger links between properties.
We also generated association rules between PS and pain properties using Link analysis (Figure 5). The link analysis among PS revealed that, the likelihood presence of phonophobia with fatigue and photophobia is very high hence this association rule has the highest confidence and lift values (lift=2.29, confidence=86.95%). Similarly, phonophobia is related with neck stiffness and photophobia with 84% confidence and 2.21 lift values. If the patients have concentration difficulty, they also have photophobia (lift=1.51, confidence=62.9%), fatigue (lift=1.51, confidence=61.29%), and neck stiffness (lift=1.25, confidence=61.29%). In addition, VAS values of the patients with dizziness, headache duration (4.676–5.32), concentration difficulties, neck stiffness, fatigue are greater than 7.79 according to their lift and confidence values (80.0%–62.33%; 1.27–0.99). In summary, the most confident association rules among symptoms and headache duration and VAS with the highest lift values have been generated for photophobia, phonophobia, fatigue, neck stiffness, dizziness, concentration difficulty. Patients with high pain VAS scores (VAS>7.793) were more likely to have symptoms of concentration difficulties, dizziness, and neck stiffness as PS.
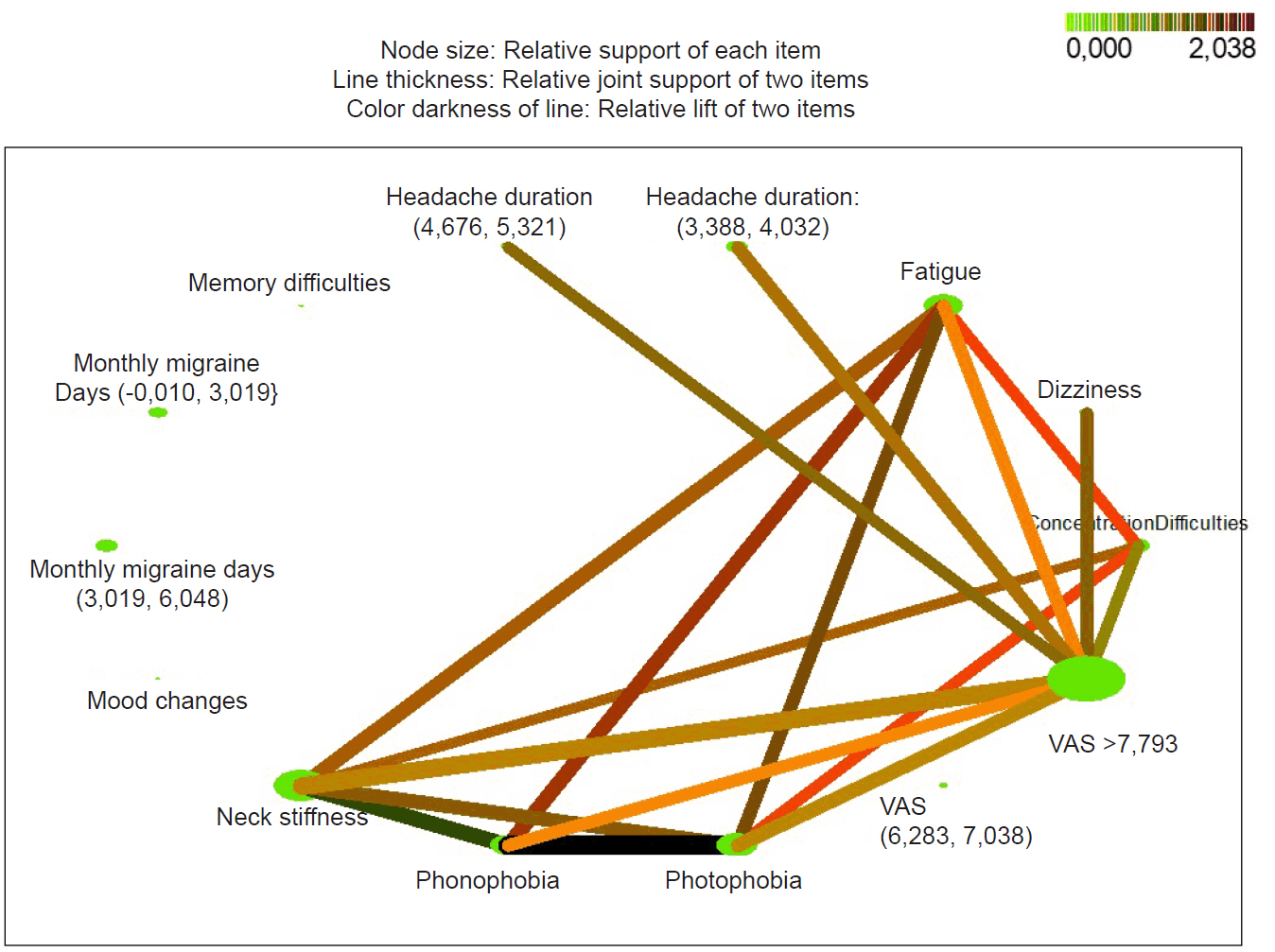
Analysis of the links among premonitory symptoms (n=139). An analysis of the links among premonitory symptoms and pain properties is given in a web graph. For premonitory symptoms, green nodes demonstrate symptom association and a darker color represents a stronger linkage between symptoms. For the relationships between visual analogue scale (VAS) scores and premonitory symptoms, darker and thicker lines and colors represent stronger associations.
3. Comparison of premonitory symptom positive and premonitory symptom negative groups
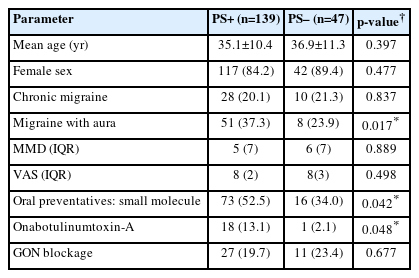
Demographic data of PS+ (n=139) and PS– (n=47) patients are given in Table 1. There is no significant difference between the number of headache days and pain severity in these two groups with similar ages and sex. The PS+ group received preventive treatment significantly higher rates compared to PS–. Although there was no difference in the number of patients using interventional treatment, the number of patients using Onabotulinumtoxin-A treatment was significantly higher in the PS+ group.
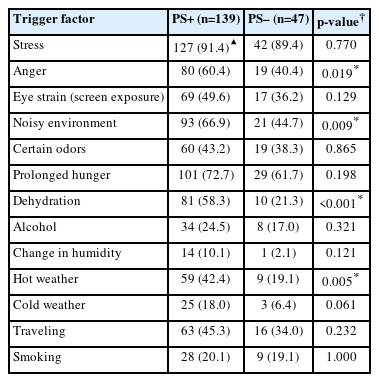
When the frequency of triggers was analyzed in PS+ and PS– patient groups, generally similar results were obtained. Noise, anger, hot weather, and dehydration triggers were significantly higher in the PS+ group. Data on triggers are summarized in Table 2.
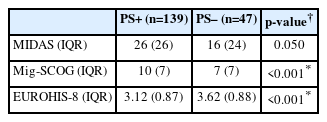
Finally, although there was no significant difference between the groups in MIDAS scores, EUROHIS-8 scores were significantly lower and MigSCOG scores were significantly higher in the PS+ group (Table 3). According to these results, it can be said that there is a decrease in the quality of life in PS+ patients without a difference in the number of headache days and the number of days of disability due to headache. Cognitive impairment during headache attacks was significantly higher in the PS+ group compared to the PS– group.
DISCUSSION
The prevalence of PS has been reported between 7.8%–100%2,6,8-12,16,26-30 in studies. Although most of these studies were conducted retrospectively, the results were observed between 33%–97%2,9,28,31,32 in prospective studies. When we look at the number of PS questioned in these studies, we see the differences. In studies in which seven or fewer symptoms were questioned, the prevalence ranged between 7.8%–42.2%,7-9 in studies in which 10 or more symptoms were questioned, between 33%–100%,16,27,31,33,34 and in studies in which 20 or more symptoms were questioned, between 21.5%–100%.10,12,28,32 A recently published study found that specifying symptoms to patients increased the prevalence of PS.26 A study by Maniyar et al.13 concluded that PS can start less than an hour before migraine or aura symptoms. We found PS rate was as high as 74.7% because we asked to the patients these PS one by one and based on ICHD-3.3 In studies using electronic diaries, PS rates were generally found to be higher at 84%–97%.2,28,32 We think that such studies provide the most reliable data on the prevalence of PS. Perhaps, if an electronic diary study and a patient interview can be conducted together, we can obtain clearer information about the prevalence.
In the PS examined, we observed that fatigue was the most common symptom, appearing in many studies, with prevalence ranging from 38%–72%.2,6,16,27-29 In our study, the most common symptom was neck stiffness. However, Lampl et al.35 reported that neck stiffness may occur at any time interval in 69% of migraine patients and Giffin et al.2 reported that the rate of prediction of migraine attack by neck stiffness was low. We believe that the high rates of neck stiffness symptoms reported by our patients may be influenced by recall bias during the interview. We think that the high rates of photophobia and phonophobia are also due to similar reasons. One study reported that the likelihood of bright light and noisy environment triggers seen in migraine is higher than the likelihood of photophobia and phonophobia seen as PS.11 Maniyar et al.13,14 mentioned that PS such as photophobia, phonophobia, and nausea overlapped with the symptoms accompanying a migraine attack. The rate of yawning, considered a pure PS,9 was determined as 14.3% in our study. In different publications, the prevalence of yawning varies between 4.2%–40%.2,9,12,16,27,28 In conclusion, our symptom prevalence data is within the range seen in the studies except for phonophobia and photophobia.
In our study, PS begin 1–6 hours before the headache attack in most participants. In one study,29 PS started 4 hours before the headache in 72% of the participants and up to 2 hours before the headache in 60% of the participants. It can be said that in a large proportion of migraineurs, PS start close to the headache. Approximately half of the patients with PS stated that the headache that started after the PS was more severe than the headache that occurred without PS. Although there is no clear data on this subject, the regions that cause PS are activated before and during the headache, and involved in pain modulation as well.9,13,36 This may explain this situation.
Our analyses show a strong association between photophobia and phonophobia. The symptoms most linked to VAS are dizziness, yawning, and frequent urination, while frequent urination and hunger are most associated with MMD. There are very few studies on PS analysis in the literature. In one study,27 it was observed that the irritability symptom was frequently associated with other symptoms. In another study,12 a strong association was found between photophobia and phonophobia, but their relationship with pain characteristics was not mentioned. Further studies on the biochemical mechanisms involved in the development of PS and the differentiation of similarities and differences in anatomical sites are needed for the analysis of these symptoms, as this will be useful for individualized treatment of migraine patients in the future.
Regarding data analysis, our study showed no significant difference between the PS+ and PS– groups in terms of MMDs, VAS, and MIDAS scores. In the literature, a positive correlation was found between PS and VAS scores in one study26 between PS+ and PS–, while no difference was observed between the groups in terms of VAS scores in other studies.26,28,29 Again, studies support that there is no difference between PS+ and PS– in terms of MMD.26-29 No difference was found between the groups in MIDAS scores in previous studies.16,23
Our data revealed that the PS+ group had significantly more use of medical preventive treatment and Onabotulinum toxin A. One study reported that preventive therapies reduced PS.28 In another study, the PS+ group used more comprehensive preventive treatment options.26
In our study, the quality of life for the PS+ group was significantly lower than that of the PS– group. Only one study has examined this situation, finding that the number of symptoms was weakly correlated with quality of life measures; however, the presence of PS showed no correlation.26
We found that cognitive impairment during a headache attack was higher in the PS+ group than in the PS– group. There is no previous study that evaluated cognitive evaluation between PS+ and PS– groups. Our observations are that we need a deeper focus on this area soon. The ictal burden of disease as measured in this study, reflects the benefits of treatment so an imbalance in rates of treatment could lead to underestimating the burden in the PS+ group prior to treatment initiation. It is also possible that the PS+ group has a greater unmeasured burden in the interictal phase. Unfortunately, we did not perform an interictal evaluation in our study though our quality of life measure may capture both ictal and interictal burden. It has been shown that interictal cognitive impairment in migraine patients may be in areas such as complex attention, executive function, immediate memory, and working memory.37,38 Recent studies revealed that, there is a co-occurrence between ictal and interictal cognitive impairments.39,40 Perhaps the increased cognitive impairment during the headache in the patient group with PS also increased in the interictal period. Increased cognitive impairment during ictal and interictal periods may explain the reduced quality of life in our PS+ patients, without affecting the number of headache days lost. More studies evaluating PS from a cognitive perspective are needed.
Migraineurs are known to have a loss of habituation41 to pain. Regions that cause PS are activated before the headache.13 These regions also modulate pain and cause pain behavior.14,19 In an electrophysiologic study in migraineurs,42 the stimulation threshold of migraineurs was found to be decreased compared to the normal population and this was associated with cortical hyperexcitability in migraineurs. Cortical hyperexcitability43,44 and interictal blood calcitonin gene-related peptide (CGRP)45 levels were found to be higher in chronic migraineurs than in episodic migraineurs. Patients with predominant PS, as in chronic migraine patients, may have increased cortical hyperexcitability compared to patients without PS. Onabotulinumtoxin-A administration was observed to decrease interictal CGRP levels.46 Interictal CGRP levels may also be higher in the PS+ group compared to the PS– group, but there is no study on this subject. The increased use of Onabotulinumtoxin-A in the PS+ group observed in our study may be for this reason. To summarize our limitations: there may be recall bias since information was gathered after the interview. We did not evaluate episodic and chronic migraine separately. Patients might confuse triggering PS with those accompanying the headache, leading to potentially inflated reports of PS.
In conclusion, the quality of life of patients with PS is more affected regardless of the severity, duration, frequency, and number of days lost due to headache. This may be related to ictal and interictal cognitive impairment, which affects PS+ patients more significantly.
Notes
AVAILABILITY OF DATA AND MATERIAL
Our raw data is organized in a spreadsheet, which can be found at the following link: https://docs.google.com/spreadsheets/d/13mPlMDtfYaT3YPvAuxdfbR4bosjAn1L4/edit?gid=1534941141#gid=1534941141
AUTHOR CONTRIBUTIONS
Conceptualization: UT, BT; Formal analysis: UT, BT; Investigation: UT, NÖG; Methodology: NÖG, AÖ; Project administration: AÖ; Supervision: NÖG, AÖ; Validation: UT, NÖG, AÖ; Visualization: UT, BT; Writing–original draft: UT, AÖ; Writing–review & editing: UT, BT, NÖG, AÖ.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING STATEMENT
Not applicable.
ACKNOWLEDGMENTS
The authors would like to sincerely thank Prof. Richard Lipton for his help in editing the manuscript.