Articles
- Page Path
- HOME > Headache Pain Res > Volume 25(1); 2024 > Article
-
Original Article
Cluster Headache Characteristics and the Severity of Obstructive Sleep Apnea: Insights from Polysomnography Analysis -
Yooha Hong1
 , Mi-Kyoung Kang1
, Mi-Kyoung Kang1 , Min Kyung Chu2
, Min Kyung Chu2 , Soo-Jin Cho1
, Soo-Jin Cho1 , Hee-Jin Im1
, Hee-Jin Im1
-
Headache and Pain Research 2024;25(1):63-71.
DOI: https://doi.org/10.62087/hpr.2024.0001
Published online: April 16, 2024
1Department of Neurology, Dongtan Sacred Heart Hospital, Hallym University College of Medicine, Hwaseong, Republic of Korea
2Department of Neurology, Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea
- Corresponding author: Hee-Jin Im, M.D., Ph.D. Department of Neurology, Dongtan Sacred Heart Hospital, Hallym University Colleage of Medicine, 7 Keunjaebong-gil, Hwaseong 18450, Republic of Korea Tel: +82-31-8086-3185, Fax: +82-31-8086-2317 E-mail: coolere@naver.com
© 2024 The Korean Headache Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 5,253 Views
- 34 Download
- 3 Crossref
Abstract
-
Purpose
- Cluster headache (CH) is characterized by circadian rhythmicity of the attacks, and it is known to respond exceptionally well to oxygen therapy. Furthermore, obstructive sleep apnea (OSA) frequently co-occurs with CH, and both conditions may be parallel outcomes of hypothalamic dysfunction rather than being causally related. The aim of this study was to analyze the association between CH characteristics and polysomnographic factors stratified by the severity of OSA in patients diagnosed with CH and OSA.
-
Methods
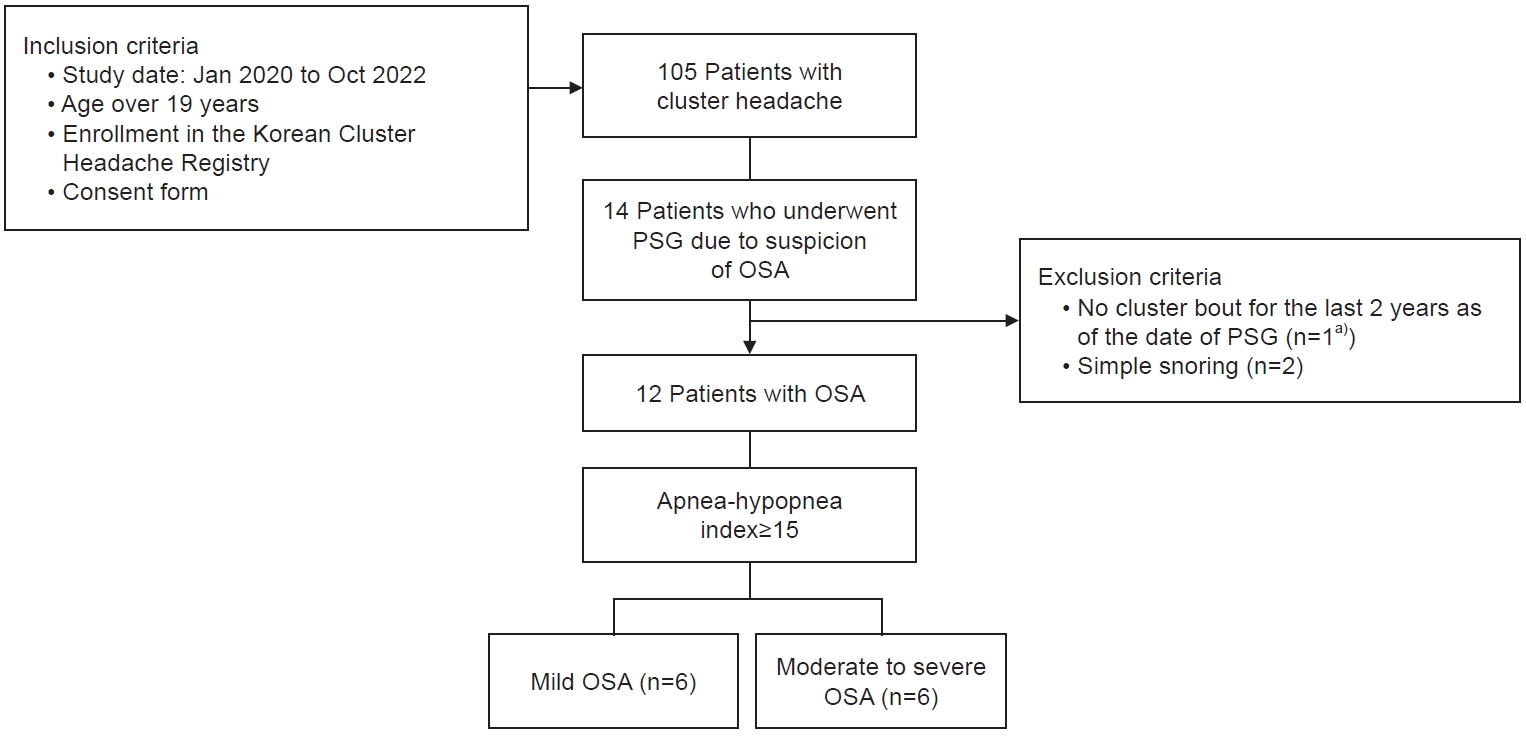
- We retrospectively analyzed the data of OSA patients with CH who were enrolled in the Korean Cluster Headache Registry and underwent polysomnography due to clinical suspicion of OSA. Basic demographic data, headache-related parameters, and polysomnographic parameters were analyzed according to the severity of OSA (apnea-hypopnea index: <15 or ≥15 per hour).
-
Results
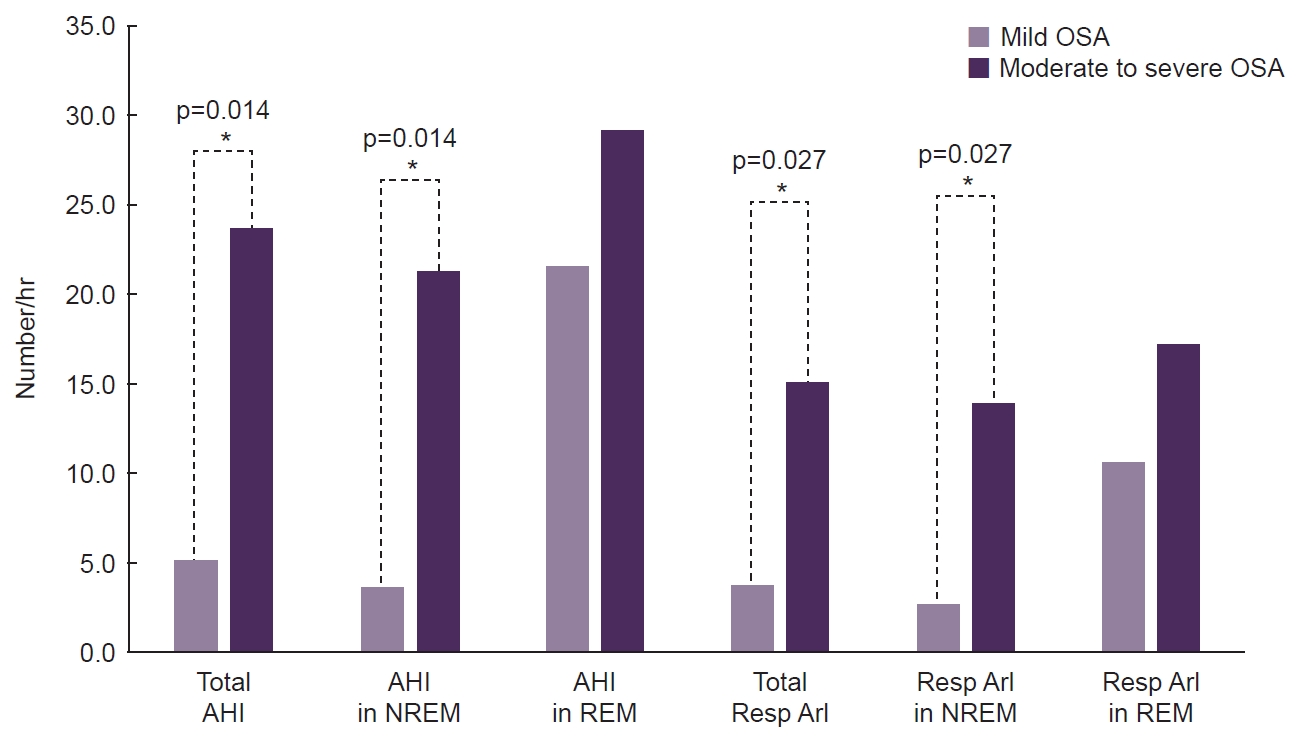
- Twelve CH patients with OSA were evaluated. The onset age of CH was higher (38.5 years vs. 19.0 years, p=0.010), and the maximal duration of cluster bouts was longer (156.5 days vs. 47.0 days, p=0.037) in the moderate-to-severe OSA group than in the mild OSA group. Unlike other polysomnographic parameters, the apnea-hypopnea index and respiratory arousal index during rapid eye movement (REM) sleep were comparable across different OSA severity levels.
-
Conclusion
- The onset age and duration of cluster bouts were associated with the severity of OSA in CH patients. Additionally, the relatively high susceptibility to hypoxia during REM sleep in patients with mild OSA implies that interventions may be potentially advantageous, even in CH patients with mild OSA.
INTRODUCTION
MATERIALS AND METHODS
RESULTS
DISCUSSION
AVAILABILITY OF DATA AND MATERIAL
Anonymized data supporting the findings presented in the current study will be shared upon reasonable request from a qualified investigator.
AUTHOR CONTRIBUTIONS
Conceptualization: SJC, HJI; Data curation: YH, MKK, SJC, HJI; Formal analysis: YH, SJC, HJI; Investigation: YH, MKK, SJC, HJI; Methodology: MKC, SJC, HJI; Software: YH, SJC; Validation: MKC, SJC, HJI; Writing–original draft: YH, MKK, HJI; Writing–review and editing: YH, MKK, MKC, SJC, HJI.
CONFLICT OF INTEREST
Soo-Jin Cho is the Editor-in-Chief of Headache and Pain Research and was not involved in the review process of this article.
Soo-Kyoung Kim is the Editor of Headache and Pain Research and was not involved in the review process of this article.
Mi-Kyoung Kang is the Editor of Headache and Pain Research and was not involved in the review process of this article.
All authors have no other conflicts of interest to declare.
FUNDING STATEMENT
Not applicable.
ACKNOWLEDGMENTS
The authors thank the Clinical Research Coordinator, Jeung-Eun Kown, for study assistance.
Values are presented as median (range) or number (%).
These p-values are based on the Mann-Whitney or Fisher exact test; *Asterisk indicates a statistically significant (p<0.05).
OSA, obstructive sleep apnea; BMI, body mass index; CH, cluster headache; HIT-6, Headache Impact Test-6; VAS, visual analog scale; PSG, polysomnography.
Values are presented as median (range) or number (%) unless otherwise specified.
These p-values are based on the Mann-Whitney or Fisher exact test; *Asterisk indicates a statistically significant (p<0.05). †These data represent the median (%).
OSA, obstructive sleep apnea; REM, rapid eye movement; NREM, non-rapid eye movement; WASO, wakefulness after sleep onset; AHI, apnea-hypopnea index; SpO2, oxygen saturation; ArI, arousal index; Resp, respiratory.
- 1. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38:1-211.ArticlePDF
- 2. Hoffmann J, May A. Diagnosis, pathophysiology, and management of cluster headache. Lancet Neurol 2018;17:75-83.ArticlePubMed
- 3. Evers S. Sleep and headache: the biological basis. Headache 2010;50:1246-1251.ArticlePubMed
- 4. Brennan KC, Charles A. Sleep and headache. Semin Neurol 2009;29:406-418.ArticlePubMedPMC
- 5. Pfaffenrath V, Pöllmann W, Rüther E, Lund R, Hajak G. Onset of nocturnal attacks of chronic cluster headache in relation to sleep stages. Acta Neurol Scand 1986;73:403-407.ArticlePubMed
- 6. Hagedorn A, Snoer A, Jensen R, Haddock B, Barloese M. The spectrum of cluster headache: a case report of 4600 attacks. Cephalalgia 2019;39:1134-1142.ArticlePubMedPDF
- 7. Barloese M, Lund N, Petersen A, Rasmussen M, Jennum P, Jensen R. Sleep and chronobiology in cluster headache. Cephalalgia 2015;35:969-978.ArticlePubMedPDF
- 8. Barloese M. Current understanding of the chronobiology of cluster headache and the role of sleep in its management. Nat Sci Sleep 2021;13:153-162.ArticlePubMedPMCPDF
- 9. Lund NL, Snoer AH, Jennum PJ, Jensen RH, Barloese MCJ. Sleep in cluster headache revisited: results from a controlled actigraphic study. Cephalalgia 2019;39:742-749.ArticlePubMedPDF
- 10. Terzaghi M, Ghiotto N, Sances G, Rustioni V, Nappi G, Manni R. Episodic cluster headache: NREM prevalence of nocturnal attacks. Time to look beyond macrostructural analysis? Headache 2010;50:1050-1054.ArticlePubMed
- 11. Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet 2014;383:736-747.ArticlePubMed
- 12. Chervin RD, Zallek SN, Lin X, Hall JM, Sharma N, Hedger KM. Sleep disordered breathing in patients with cluster headache. Neurology 2000;54:2302-2306.ArticlePubMed
- 13. Graff-Radford SB, Newman A. Obstructive sleep apnea and cluster headache. Headache 2004;44:607-610.ArticlePubMedPDF
- 14. Nobre ME, Leal AJ, Filho PM. Investigation into sleep disturbance of patients suffering from cluster headache. Cephalalgia 2005;25:488-492.ArticlePubMedPDF
- 15. Evers S, Barth B, Frese A, Husstedt IW, Happe S. Sleep apnea in patients with cluster headache: a case-control study. Cephalalgia 2014;34:828-832.ArticlePubMedPDF
- 16. Graff-Radford SB, Teruel A. Cluster headache and obstructive sleep apnea: are they related disorders? Curr Pain Headache Rep 2009;13:160-163.ArticlePubMedPDF
- 17. El Shayeb M, Topfer LA, Stafinski T, Pawluk L, Menon D. Diagnostic accuracy of level 3 portable sleep tests versus level 1 polysomnography for sleep-disordered breathing: a systematic review and meta-analysis. CMAJ 2014;186:E25-E51.ArticlePubMedPMC
- 18. Cho S, Cho SJ, Lee MJ, et al. Clinical characteristics of pre-attack symptoms in cluster headache: a large series of Korean patients. Cephalalgia 2021;41:227-236.ArticlePubMedPDF
- 19. Yang M, Rendas-Baum R, Varon SF, Kosinski M. Validation of the Headache Impact Test (HIT-6™) across episodic and chronic migraine. Cephalalgia 2011;31:357-367.ArticlePubMedPMCPDF
- 20. Aicher B, Peil H, Peil B, Diener HC. Pain measurement: Visual Analogue Scale (VAS) and Verbal Rating Scale (VRS) in clinical trials with OTC analgesics in headache. Cephalalgia 2012;32:185-197.ArticlePubMed
- 21. Malhotra A, Ayappa I, Ayas N, et al. Metrics of sleep apnea severity: beyond the apnea-hypopnea index. Sleep 2021;44:zsab030.ArticlePubMedPMCPDF
- 22. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540-545.ArticlePubMed
- 23. Chung F, Abdullah HR, Liao P. STOP-Bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest 2016;149:631-638.ArticlePubMed
- 24. Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2001;2:297-307.ArticlePubMed
- 25. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193-213.ArticlePubMed
- 26. Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev 2017;34:70-81.ArticlePubMed
- 27. Weintraub JR. Cluster headaches and sleep disorders. Curr Pain Headache Rep 2003;7:150-156.ArticlePubMedPDF
- 28. Chung PW, Kim BS, Park JW, et al. Smoking history and clinical features of cluster headache: results from the Korean Cluster Headache Registry. J Clin Neurol 2021;17:229-235.ArticlePubMedPMCPDF
- 29. Ofte HK, Berg DH, Bekkelund SI, Alstadhaug KB. Insomnia and periodicity of headache in an arctic cluster headache population. Headache 2013;53:1602-1612.ArticlePubMed
- 30. McDonough P, Moffatt RJ. Smoking-induced elevations in blood carboxyhaemoglobin levels. Effect on maximal oxygen uptake. Sports Med 1999;27:275-283.ArticlePubMed
- 31. Wagner PD. Muscle O2 transport and O2 dependent control of metabolism. Med Sci Sports Exerc 1995;27:47-53.ArticlePubMed
- 32. Barloese M, Jennum P, Knudsen S, Jensen R. Cluster headache and sleep, is there a connection? A review. Cephalalgia 2012;32:481-491.ArticlePubMedPDF
- 33. Nobre ME, Filho PF, Dominici M. Cluster headache associated with sleep apnoea. Cephalalgia 2003;23:276-279.ArticlePubMedPDF
- 34. Mokhlesi B, Punjabi NM. “REM-related” obstructive sleep apnea: an epiphenomenon or a clinically important entity? Sleep 2012;35:5-7.ArticlePubMedPMC
- 35. Nath Zallek S, Chervin RD. Improvement in cluster headache after treatment for obstructive sleep apnea. Sleep Med 2000;1:135-138.ArticlePubMed
- 36. Song MJ, Cho SJ, Im HJ. Improvement of nocturnal cluster headache after continuous positive airway pressure therapy in a patient with severe obstructive sleep apnea. J Sleep Med 2021;18:55-57.Article
REFERENCES
Figure & Data
References
Citations

- Morning Headaches: An In-depth Review of Causes, Associated Disorders, and Management Strategies
Yooha Hong, Mi-Kyoung Kang, Min Seung Kim, Heejung Mo, Rebecca C. Cox, Hee-Jin Im
Headache and Pain Research.2025; 26(1): 66. CrossRef - Inverse association of obesity with bout periodicity in episodic cluster headache: a multicenter cross-sectional study
Byung-Su Kim, Mi Ji Lee, Byung-Kun Kim, Jong-Hee Sohn, Tae-Jin Song, Min Kyung Chu, Soo-Kyoung Kim, Jeong Wook Park, Heui-Soo Moon, Pil-Wook Chung, Soo-Jin Cho
The Journal of Headache and Pain.2025;[Epub] CrossRef - Subtype shift, relapse rate and risk factors of frequent relapse in cluster headache: A multicenter, prospective, longitudinal observation
Mi Ji Lee, Soo-Kyoung Kim, Min Kyung Chu, Jae Myun Chung, Heui-Soo Moon, Pil-Wook Chung, Jeong Wook Park, Byung-Kun Kim, Kyungmi Oh, Yun-Ju Choi, Jong-Hee Sohn, Byung-Su Kim, Dae Woong Bae, Daeyoung Kim, Tae-Jin Song, Kwang-Yeol Park, Soo-Jin Cho
Cephalalgia.2025;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
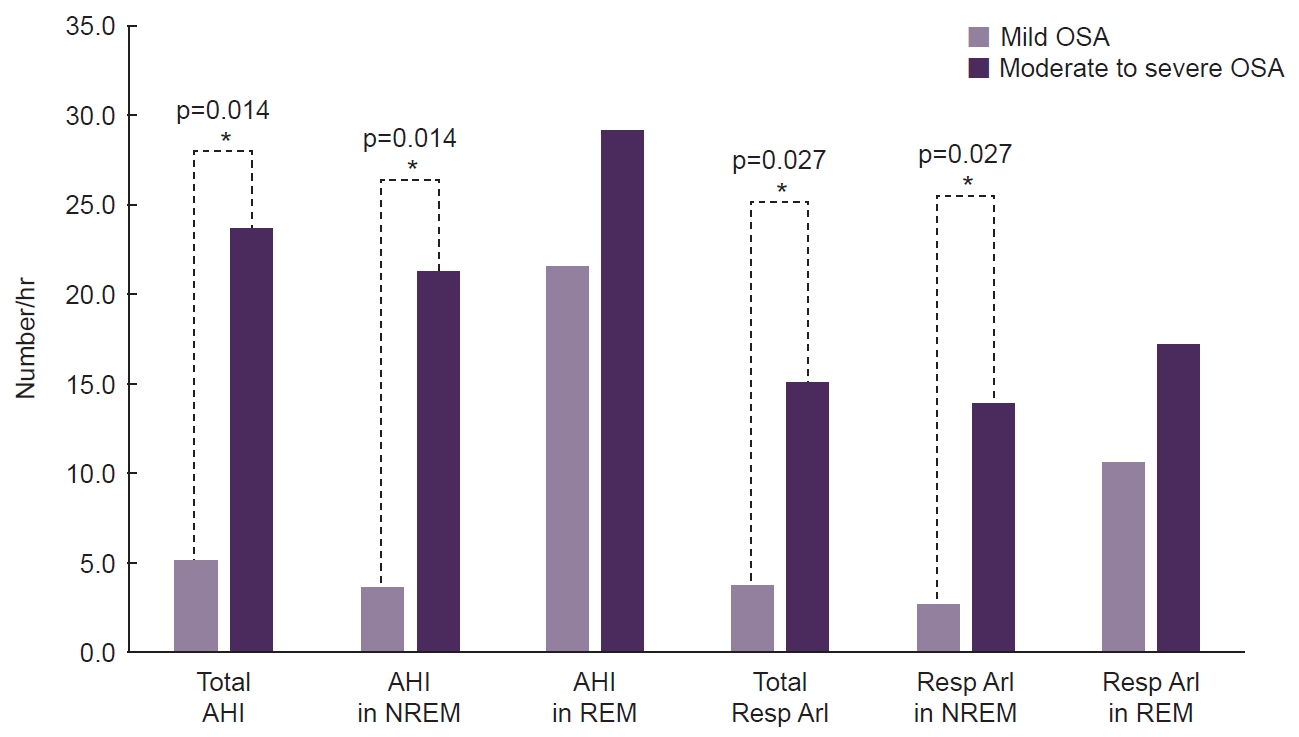
Figure 1.
Figure 2.
| Characteristic | Mild OSA (n=6) | Moderate to severe OSA (n=6) | p-value |
|---|---|---|---|
| Age (yr) | 40.5 (24.0–42.0) | 43.5 (34.0–62.0) | 0.076 |
| Male sex | 6 (100.0) | 6 (100.0) | >0.99 |
| BMI (kg/m2) | 26.6 (23.8–31.4) | 26.0 (21.0–32.9) | 0.631 |
| Hypertension | 1 (16.7) | 3 (50.0) | 0.545 |
| Diabetes | 1 (16.7) | 0 (0.0) | >0.99 |
| Hyperlipidemia | 4 (66.7) | 4 (66.7) | >0.99 |
| Current smoking | 3 (50.0) | 5 (83.3) | 0.105 |
| Age at diagnosis of CH (yr) | 28.0 (23.0–40.0) | 41.5 (27.0–55.0) | 0.044* |
| Age at onset of CH (yr) | 19.0 (15.0–31.0) | 38.5 (25.0–55.0) | 0.010* |
| HIT-6 score | 68.5 (63.0–78.0) | 70.0 (48.0–78.0) | 0.872 |
| Maximal duration of cluster bouts (day) | 47.0 (33.0–128.0) | 156.5 (67.0–1,247.0) | 0.037* |
| VAS score (headache severity) | 9.0 (7.0–10.0) | 9.0 (5.0–10.0) | 0.672 |
| Duration of CH attacks (min) | 110.0 (30.0–180.0) | 35.0 (20.0–120.0) | 0.319 |
| Frequency of CH attacks (day) | 1.0 (0.5–2.5) | 1.8 (1.0–2.0) | 0.360 |
| Location of CH attacks (right/left) | 4 (66.7)/2 (33.3) | 1 (16.7)/5 (83.3) | 0.242 |
| Presence of circadian rhythm in CH attacks | 5 (83.3) | 5 (83.3) | >0.99 |
| CH attacks only at night | 1 (16.7) | 3 (50.0) | 0.545 |
| Presence of morning headache | 1 (16.7) | 1 (16.7) | >0.99 |
| Improvement in response to oxygen therapy | 1 (16.7) | 4 (66.7) | 0.143 |
| Improvement in response to triptan therapy | 5 (83.3) | 4 (66.7) | 0.211 |
| PSG during cluster bouts | 4 (66.7) | 5 (83.3) | >0.99 |
| Variable | Mild OSA (n=6) | Moderate to severe OSA (n=6) | p-value |
|---|---|---|---|
| Excessive sleep sleepiness | 5.0 (2.0–11.0) | 5.0 (2.0–15.0) | 0.421 |
| Insomnia Severity Index | 10.5 (4.0–23.0) | 8.5 (5.0–17.0) | 0.345 |
| Moderate to severe insomnia | 2 (33.3) | 1 (16.7) | 0.500 |
| Pittsburgh Sleep Quality Index | 8.0 (8.0–16.0) | 9.5 (8.0–14.0) | 0.809 |
| Total sleep time (min) | 375.7 (350.6–420.7) | 367.1 (317.5–383.3) | 0.423 |
| REM sleep (min)† | 28.8 (7.8) | 30.5 (9.3) | 0.423 |
| NREM sleep (min)† | 351.3 (92.3) | 328.0 (90.8) | 0.423 |
| Sleep efficiency (%) | 94.4 (81.9–98.9) | 95.8 (81.9–98.2) | 0.936 |
| WASO (min) | 12.1 (4.0–83.0) | 12.5 (6.5–70.0) | 0.873 |
| Total AHI (events/hr) | 5.7 (5.0–9.4) | 22.3 (16.1–53.3) | 0.004* |
| AHI in REM (events/hr) | 23.5 (0.0–30.7) | 24.6 (5.3–58.5) | 0.688 |
| AHI in NREM (events/hr) | 4.3 (3.4–9.4) | 19.0 (14.6–56.1) | 0.004* |
| REM/NREM-AHI ratio | 5.8 (0.0–6.8) | 0.9 (0.2–4.0) | 0.054 |
| Mean SpO2 (%) | 96.2 (95.3–97.4) | 94.6 (93.2–96.2) | 0.036* |
| Minimum SpO2 (%) | 90.5 (70.0–92.0) | 86.5 (69.0–89.0) | 0.091 |
| Total ArI | 14.1 (6.4–33.4) | 21.3 (18.8–51.4) | 0.037* |
| Resp ArI | 4.6 (1.6–8.9) | 14.6 (8.0–50.1) | 0.004* |
| Total ArI in REM | 16.6 (0.0–26.2) | 20.0 (0.0–66.0) | 0.688 |
| Resp ArI in REM | 14.3 (0.0–21.4) | 14.4 (0.0–45.9) | 0.872 |
| Total ArI in NREM | 13.4 (5.7–33.4) | 20.9 (17.9–53.2) | 0.037* |
| Resp ArI in NREM | 3.4 (0.8–8.9) | 13.4 (8.6–51.7) | 0.006* |
Values are presented as median (range) or number (%). These p-values are based on the Mann-Whitney or Fisher exact test; *Asterisk indicates a statistically significant (p<0.05). OSA, obstructive sleep apnea; BMI, body mass index; CH, cluster headache; HIT-6, Headache Impact Test-6; VAS, visual analog scale; PSG, polysomnography.
Values are presented as median (range) or number (%) unless otherwise specified. These p-values are based on the Mann-Whitney or Fisher exact test; *Asterisk indicates a statistically significant (p<0.05). †These data represent the median (%). OSA, obstructive sleep apnea; REM, rapid eye movement; NREM, non-rapid eye movement; WASO, wakefulness after sleep onset; AHI, apnea-hypopnea index; SpO2, oxygen saturation; ArI, arousal index; Resp, respiratory.
Table 1.
Table 2.
TOP
 KHS
KHS