Cluster Headache Characteristics and the Severity of Obstructive Sleep Apnea: Insights from Polysomnography Analysis
Article information
Abstract
Purpose
Cluster headache (CH) is characterized by circadian rhythmicity of the attacks, and it is known to respond exceptionally well to oxygen therapy. Furthermore, obstructive sleep apnea (OSA) frequently co-occurs with CH, and both conditions may be parallel outcomes of hypothalamic dysfunction rather than being causally related. The aim of this study was to analyze the association between CH characteristics and polysomnographic factors stratified by the severity of OSA in patients diagnosed with CH and OSA.
Methods
We retrospectively analyzed the data of OSA patients with CH who were enrolled in the Korean Cluster Headache Registry and underwent polysomnography due to clinical suspicion of OSA. Basic demographic data, headache-related parameters, and polysomnographic parameters were analyzed according to the severity of OSA (apnea-hypopnea index: <15 or ≥15 per hour).
Results
Twelve CH patients with OSA were evaluated. The onset age of CH was higher (38.5 years vs. 19.0 years, p=0.010), and the maximal duration of cluster bouts was longer (156.5 days vs. 47.0 days, p=0.037) in the moderate-to-severe OSA group than in the mild OSA group. Unlike other polysomnographic parameters, the apnea-hypopnea index and respiratory arousal index during rapid eye movement (REM) sleep were comparable across different OSA severity levels.
Conclusion
The onset age and duration of cluster bouts were associated with the severity of OSA in CH patients. Additionally, the relatively high susceptibility to hypoxia during REM sleep in patients with mild OSA implies that interventions may be potentially advantageous, even in CH patients with mild OSA.
INTRODUCTION
Cluster headache (CH) is a rare and severely disabling trigeminal autonomic cephalalgia with strong chronobiological traits.1 CH, characterized by bouts and remission periods, can be classified as episodic or chronic. Distinguishing from other headache disorders, oxygen therapy has proven effective for rescuing CH attack.2
CH is considered one of the primary headache disorders most closely associated with sleep.3 In general, headaches and sleep are intertwined in various aspects, including clinical, physiological, epidemiological, and anatomical aspects.4 Evidence supports that CH attacks are more prevalent and severe during the night compared to daytime attacks in patients with periodicity (occurring at specific times during day or seasons).5-7 Initial studies suggested that CH attacks are associated with rapid eye movement (REM) sleep, coinciding with the onset of the first REM sleep phase within 90 minutes of falling sleep. However, recent several studies have shown that CH attacks are not significantly associated with REM sleep.8-10
Obstructive sleep apnea (OSA) is one of the most common sleep disorder that causes recurrent hypoxia and frequent arousal due to repeated airway collapse.11 CH has been linked with sleep disordered breathing, particularly OSA. The higher prevalence of OSA (from 58%–80%) among CH patients compared to healthy controls, coupled with the increased frequency of CH attacks at night, led to the hypothesis of a potential causal relationship between these two conditions.12-14 Studies conducted on CH patients showed an increased prevalence of OSA syndrome and a higher respiratory distress index during CH attack compared to healthy controls.15 However, the true relationship between CH and OSA is complex and has not been clarified yet. Currently, OSA and CH may be considered as parallel outcomes of hypothalamic dysfunction rather than a causal relationship.16
In this complex relationship between CH and sleep, our study analyzed the association of CH characteristics and polysomnographic factors stratified by OSA severity in patients with CH and diagnosed with OSA. The aim was to explore the correlation between CH and sleep, specifically focusing on their association with OSA.
MATERIALS AND METHODS
1. Ethics approval and consent to participate
All participants provided written informed consent when they enrolled in the KCHR (No. 2016-09-396). The study design was approved by the Institutional Review Board of Dongtan Sacred Heart Hospital of Hallym University and allowed of additional written informed consent to be waived due to retrospective data collection and fully anonymity (No. 2022-12-003-001). All methods were performed in accordance with the relevant guidelines and regulations.
2. Participants
This retrospective, observational single-center study included participants with CH who underwent polysomnography (PSG) on clinical suspicion of OSA at the headache clinic in the Department of Neurology of the Hallym University Dongtan Sacred Hospital (Hwaseong, Korea) between January 2020 and October 2022. CH was diagnosed by two neurologists (SJ Cho and HJ Im) based on individual interviews and the diagnostic criteria of the 3rd edition of the International Classification of Headache Disorder.1 PSG was conducted as a level 1 sleep study (i.e., an overnight stay in a sleep laboratory with a technician in attendance)17 at the Sleep Center of Hallym University Dongtan Sacred Hospital.
The inclusion criteria for participants were as follows: 1) participation in the Korean Cluster Headache Registry (KCHR), a prospective observational study of CH,18 2) being over 19 years of age, 3) and having undergone PSG between January 2020 and October 2022 on suspicion of OSA. The exclusion criteria were as follows: 1) PSG conducted more than 2 years after the last cluster bout, 2) failure to be diagnosed with OSA by PSG. In the 3rd edition of the International Classification of Headache Disorders,1 CH is classified as episodic (i.e., bouts lasting from 7 days to 1 year, separated by out-of-bout periods lasting at least 3 months) or chronic (i.e., bouts lasting 1 year or longer without remission, or with out-of-bout periods lasting less than 3 months) CH, and our study investigated both episodic and chronic CH. All participants were interviewed and evaluated by trained personnel using a self-reported questionnaire.
3. Assessment of cluster headache
All participants in the KCHR completed a structured questionnaire designed for the evaluation of CH. The KCHR protocol evaluated sociodemographic variables, including sex, ages at onset and presentation, body mass index (BMI), and history of smoking and alcohol consumption. We collected the following clinical data regarding current and previous cluster bout including headache characteristics, disease duration, headache frequency (headache attacks per day), headache intensity, headache duration, diurnal rhythmicity, total number of bouts that was independent of disease duration, and presence of premorbid migraine. The following evaluations were also conducted: the Headache Impact Test-6 used for measuring the impact of a headache,19 and the visual analog scale (VAS) used for measuring the pain intensity of a headache.20 The VAS score is determined by measuring the distance (mm) on the 10-cm line between the “no pain” anchor and the patient’s mark. A higher score indicates greater pain intensity.
4. Assessment of sleep factors and polysomnography data
All participants underwent laboratory nocturnal PSG (Embla®; Natus), which entails electroencephalogram, electrooculogram, electromyogram, electrocardiogram, respiratory flow and effort, oximetry, pulse, and body position recordings. PSG data were reviewed and scored by sleep experts using the Embla RemLogic PSG Software using 30-second epochs according to the standard criteria by the American Academy of Sleep Medicine. Standard sleep indexes including total sleep time, sleep efficiency, wake after sleep onset, sleep latency, percentage of each sleep stage over total sleep time, apnea-hypopnea index (AHI, total number of apneas plus hypopneas per hour of sleep), mean oxygen saturation, lowest oxygen saturation, total arousals, and an arousal index were calculated. OSA has been defined and quantified primarily by the frequency of apneas and hypopneas during sleep. According to the International Classification of Sleep Disorders 3rd edition criteria, the severity of OSA by AHI is divided into mild (≥5 to 15 per hour), moderate (≥15 to 30 per hour), and severe (≥30 per hour).21 In this study, the participants were divided into two groups according to the score of AHI: a mild OSA group (AHI≥5 to 15 per hour) and a moderate to severe OSA group (AHI≥15 per hour). Sleep parameters were evaluated using a self-reported questionnaire booklet. Excessive daytime sleepiness was defined as an Epworth Sleepiness Scale score≥11.22 The likelihood of OSA was assessed by the STOP-Bang questionnaire. A score over 3 reflected a moderate risk of apnea during sleep.23 And, insomnia symptoms were assessed using the Insomnia Severity Index, with a score>14 indicating moderate-to-severe insomnia.24 Sleep quality was evaluated using the Pittsburgh Sleep Quality Index with a score>5 indicating poor sleep quality.25
5. Statistical analysis
Categorical data were presented as frequencies and percentages. Continuous data, such as age, daily headache frequency, and headache intensity were presented as the median with data range (minimum to maximum). Nonparametric tests were used to establish statistical significance at p<0.05 when the normality assumption was not met. In our study, due to the small sample size and non-normal distribution of the data, group comparisons according to OSA severity were performed using nonparametric methods. Specifically, the Mann-Whitney test was used for continuous variables, and Fisher’s exact test was used for categorical variables. Data analysis was performed using SPSS version 24 (IBM Corp.).
RESULTS
1. Participants
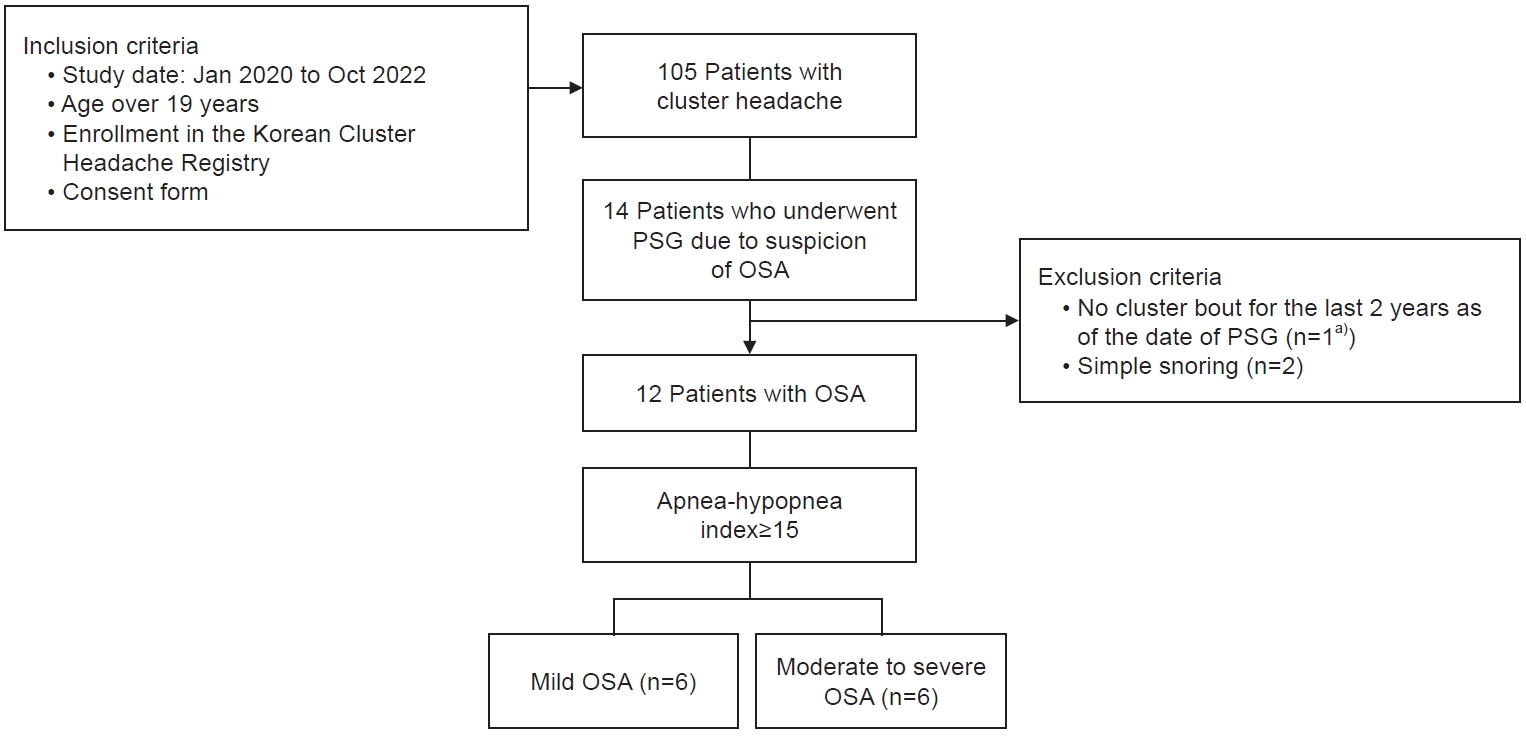
Among 105 participants in the KCHR for 34 months, 14 participants who were clinically suspicious of OSA with CH underwent PSG. However, two participants were excluded due to being undiagnosed with OSA (AHI<5 per hour) and no cluster bout for 2 years after PSG. Ultimately, a total of 12 participants were included in our analysis (Figure 1). All participants were male, and the median age was 42 years (range, 24–62 years). The median BMI was 26.1 kg/m2 (range, 21.0–32.9 kg/m2). There were 10 participants (83.3%) with episodic CH and two participants (16.7%) with chronic CH. Nine participants (75.0%) had undergone PSG during the cluster bout, and three participants had undergone PSG during the remission period. Six participants were classified for mild OSA, and the remaining participants were for moderate to severe OSA (AHI≥15 per hour). Two patients with chronic CH were classified as having moderate to severe OSA.
2. Clinical characteristics of cluster headache according to obstructive sleep apnea severity
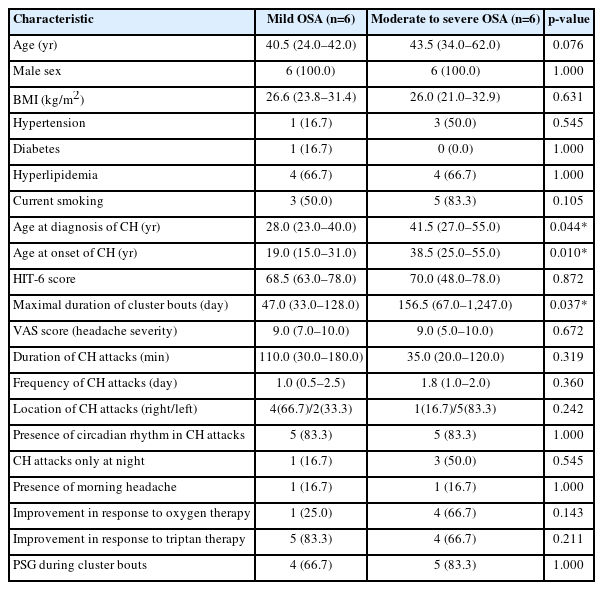
The median disease duration of CH in all participants was 6 years (range, 1–16 years), and the median onset age of CH was 29 years (range, 15–55 years). The age at onset of CH in the moderate to severe OSA group was higher than in the mild OSA group (median, 38.5 years vs. 19.0 years; p=0.010). The maximal duration of cluster bout in the moderate to severe group (median, 156.5 days) was longer than that in the mild OSA group (median, 47.0 days; p=0.037). Other clinical features of cluster attacks including intensity, duration, and frequency did not show significant differences and were comparable between CH patients with moderate to severe OSA and those with mild OSA (Table 1). A total of 10 participants (83.3%) reported diurnal periodicity. There were no statistically significant differences in age, sex, BMI, and cardiovascular risk factors including hypertension, diabetes, hyperlipidemia, and smoking between the two groups.
3. Sleep parameters and polysomnography data features according to obstructive sleep apnea severity in cluster headache patients
In this study, most participants (n=9, 75.0%) had undergone a PSG during the cluster bout (ictal phase of CH) and no cluster attack was observed during PSG. As for the difference of OSA severity between two groups, the median value of mean oxygen saturation was 96.2% (range, 95.3%–97.4%) in the mild OSA group and 94.6% (range, 93.2%–96.2%) in the moderate to severe OSA group. There was a significant difference in AHI scores between the two groups during non-rapid eye movement (NREM) sleep, as well (4.3 vs. 19.0, p=0.004). However, there was no significant difference in AHI during REM sleep according to OSA severity (23.5 vs. 24.6, p=0.688). As a result, the REM-AHI/NREM-AHI ratio was higher in the mild group than in the moderate to severe group with borderline significance (5.8 vs. 0.9, p=0.054). Lowest saturation and longest apnea showed no significant differences between the two groups. As shown in Table 2, there was a significant difference in respiratory arousal index according to OSA severity during NREM sleep (13.4 vs. 20.9, p=0.037). During REM sleep stage, however, respiratory arousal index between two groups were comparable (14.3 vs. 14.4, p=0.872). And these results were also demonstrated in an analysis for patients who undergone PSG during the cluster bout (Figure 2). As shown in Table 2, there was no significant difference between the two groups in moderate to severe insomnia and poor sleep quality. Among the 12 patients included in the final analysis, only three patients were diagnosed with moderate to severe insomnia.

Polysomnographic data and sleep parameters of cluster headache patients (n=12) according to OSA severity
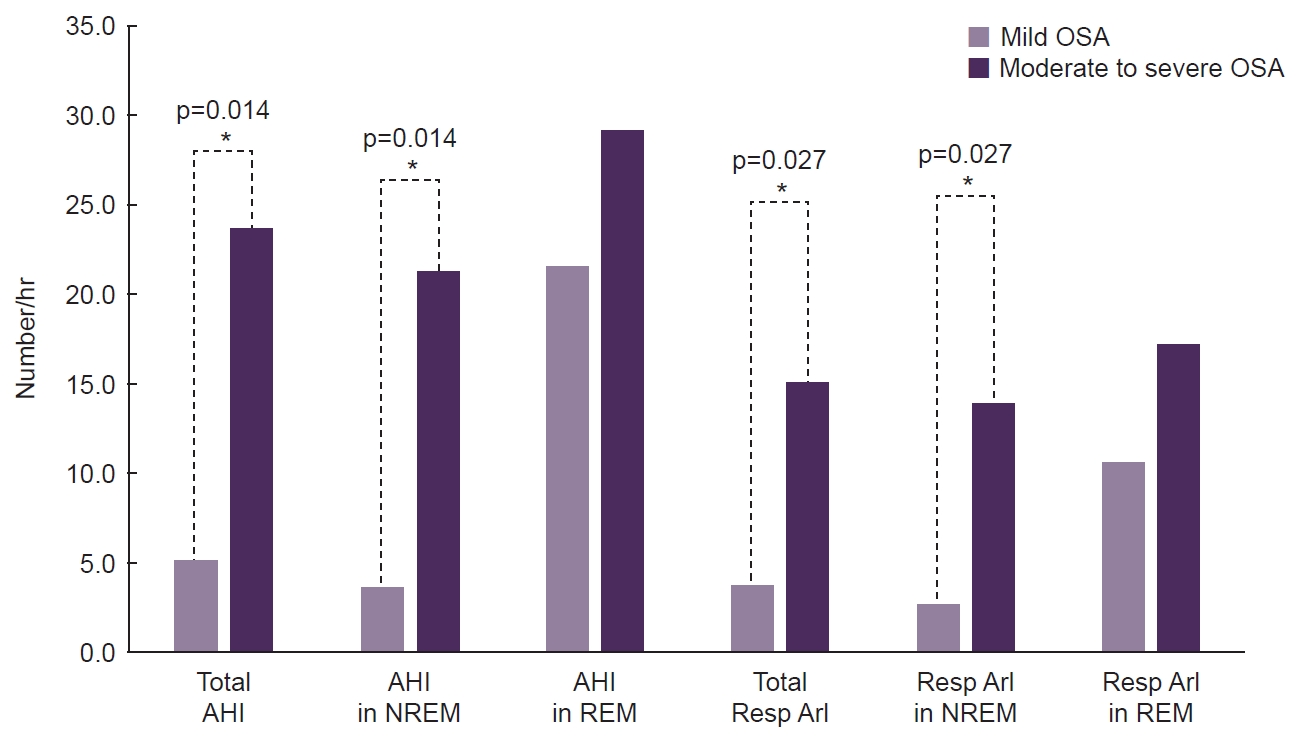
Comparison of polysomnographic respiratory parameters according to OSA severity in patients during cluster bouts.
*Asterisk indicates a statistically significant (p<0.05).
AHI, apnea-hypopnea index; NREM, non-rapid eye movement; REM, rapid eye movement; Resp, respiratory; ArI, arousal index; OSA, obstructive sleep apnea.
DISCUSSION
This is the first study that evaluated the characteristics of CH and polysomnographic parameters according to OSA severity in daily clinical practice using a prospective CH registry. We used single hospital data of patients with CH from the past 34 months. In the dataset 13.3% of the CH patients had undergone PSG, and 85.7% had been diagnosed with OSA. In previous study, OSA were reported to be diagnosed with PSG in 29% among the patients with CH compared to 7% among age–sex-matched healthy controls.15 Compared to the prevalence (6%–17%) of OSA in the general population, the CH population has a relatively higher prevalence in OSA, so active screen for OSA is recommended in CH patients.13,26
Moderate to severe OSA accounted for half of the diagnosed OSA in participants with CH in our study, which is slightly lower than the 60% reported in previous studies of CH.27 Interestingly, the median onset age of CH was significantly higher in CH patients with moderate to severe OSA group than in those with mild OSA group (38.5 years vs. 19.0 years), while in the multicenter data in Korea, the mean onset age of CH was 29.3 years28 and the peak onset age was around 30 years.29 Because the severity of OSA depends on increasing age,26 this association can be consequential a coincidence, but detrimental effect of moderate to severe OSA might have a role for occurrence of CH.30,31
In addition, the maximal duration of cluster bout during the follow-up period was significantly longer in CH patients with moderate to severe OSA than in those with mild OSA. Two patients with chronic CH were classified as having moderate to severe OSA. These results support the previously reported association between moderate to severe OSA and refractory or chronic CH, which was not present in mild OSA.32,33 Therefore, the findings that the older onset age and the longer duration of cluster bout, the higher severity of OSA, which means that there is relationship and comorbidity, at least, between CH and OSA.
In this study, there were no differences in AHI and respiratory arousal index during the REM sleep stage according to OSA severity (Table 2, Figure 2). This indicates an increased susceptibility to hypoxia during REM sleep, especially among those with mild OSA, resulting in REM-related OSA marked by respiratory difficulties during this phase of sleep. Particularly, REM sleep in OSA patients is associated with more frequent and prolonged obstructive events, often accompanied by severe oxyhemoglobin desaturation.34 This increased vulnerability during REM sleep is attributed to reduced pharyngeal muscle activity due to withdrawal of excitatory inputs to upper airway motor neurons.34 Consequently, the upper airway is more prone to collapse during REM sleep in OSA patients compared to NREM sleep. Even in cases of mild OSA, REM sleep can contribute to a cycle of heightened respiratory distress and frequent arousals in patients with CH. Therefore, interventions such as positive airway pressure (PAP) therapy may be beneficial for CH patients experiencing OSA symptoms, such as excessive daytime sleepiness, snoring, or stopped breathing during sleep. Case reports35,36 have demonstrated symptom improvement in CH patients following PAP therapy, suggesting its potential benefits in managing CH when OSA coexists. This underscores the importance of considering sleep-related factors in CH management.
Our study has certain limitations. First, the details about CH were collected via self-reports from the participants, which could have resulted in an over or underestimation of the true clinical characteristics of the CH attacks. Second, the sample size of this single-center study was small, so selection bias and type I or type II errors might have been committed. It is difficult to obtain large sample sized as CH patients presents with disabling pain when they visit the hospital, making it difficult to evaluate a sleep disorder and conduct laboratory PSG, especially during the cluster bout. Third, the low prevalence (11.4%) of OSA in our CH registry could have been influenced by the low performance of PSG.
It was difficult to perform sleep study for the patient, especially those during cluster bout with severe pain. However, it may have also reflected the real-world proportion of OSA evaluation by PSG among consecutive CH patients. Future studies on the appropriate indications for PSG and intervention of OSA in patients with CH are warranted. Therefore, a multicenter prospective randomized study regarding impacts on CH in patients currently using PAP as treatment of OSA is needed.
In conclusion, onset age of CH and maximal duration of cluster bout were both associated with severity of OSA in CH patients. And the relatively high susceptibility of sleep apnea during REM sleep in patients with mild OSA suggests the potential benefits of intervention even in mild severity of OSA, for CH patients. Although the relationship of OSA and CH is not causal, considering PSG examinations for comorbid OSA and can be beneficial for further management, especially in patients with CH who are elderly, experience prolonged cluster bouts, or exhibit unresponsiveness to drug treatment.
Notes
AVAILABILITY OF DATA AND MATERIAL
Anonymized data supporting the findings presented in the current study will be shared upon reasonable request from a qualified investigator.
AUTHOR CONTRIBUTIONS
Conceptualization: SJC, HJI; Data curation: YH, MKK, SJC, HJI; Formal analysis: YH, SJC, HJI; Investigation: YH, MKK, SJC, HJI; Methodology: MKC, SJC, HJI; Software: YH, SJC; Validation: MKC, SJC, HJI; Writing–original draft: YH, MKK, HJI; Writing–review and editing: YH, MKK, MKC, SJC, HJI.
CONFLICT OF INTEREST
Soo-Jin Cho is the Editor-in-Chief of Headache and Pain Research and was not involved in the review process of this article.
Soo-Kyoung Kim is the Editor of Headache and Pain Research and was not involved in the review process of this article.
Mi-Kyoung Kang is the Editor of Headache and Pain Research and was not involved in the review process of this article.
All authors have no other conflicts of interest to declare.
FUNDING STATEMENT
Not applicable.
ACKNOWLEDGMENTS
The authors thank the Clinical Research Coordinator, Jeung-Eun Kown, for study assistance.