Abstract
- Migraine, a prevalent neurological disorder, is more common in women than in men. This sex difference is more pronounced after menarche and diminishes after menopause. Migraines in women are influenced by the menstrual cycle, pregnancy, and lactation, suggesting a connection to sex hormones, known as the estrogen withdrawal theory. Beyond endogenous hormonal changes accompanying reproductive events, exogenous hormonal factors such as contraceptives or hormone replacement therapy may also affect migraines. The hormonal influence cannot be explained simply by serum estrogen levels; instead, it involves a complex interplay of various factors. Here, we delineate aspects of migraines associated with endogenous and exogenous hormonal changes over the course of a woman’s life, exploring the mechanisms and contributing factors through which sex hormones influence migraines.
-
Keywords: Gonadal steroid hormone, Migraine disorder, Reproductive history
INTRODUCTION
Migraines are a prevalent neurological disorder that significantly affect daily life and pose substantial socioeconomic burdens. More than 10% of the general population experiences migraines, with a higher prevalence reported among women.1 Sexual differences in migraine prevalence become more pronounced after puberty and decrease after menopause, and sex hormones are known to play a role.2 The well-established phenomenon of menstrual migraine, which is characterized by headaches induced by a decrease in estrogen levels during the late luteal phase, is known as the “estrogen withdrawal theory.”3 Estrogen also plays a crucial role in modulating excitatory and inhibitory pain neurotransmission.4 These findings suggest that hormonal factors can significantly influence the sexual differences observed in migraine.
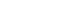
Women experience various endogenous and exogenous reproductive events, including menstruation, pregnancy, breastfeeding, menopause, contraception, and hormone replacement treatment (Figure 1). These events can induce rapid and variable changes in hormonal status, potentially influencing migraine symptoms and prevalence. Consequently, understanding the association between hormones and migraines will be essential to any investigation of the mechanisms and treatment of migraines. This review describes the intricate relationship between migraines and hormones in women. Also, this review describes the hormonal changes in response to reproductive events throughout a woman’s life and how these changes contribute to patterns in migraine occurrence.
SEX DIFFERENCES IN MIGRAINE EPIDEMIOLOGY AND SYMPTOMS
The prevalence of migraines is reportedly three times higher in women than in men.5 According to the American Migraine Prevalence and Prevention study, the overall 1-year migraine prevalence is of 11.7%, with women experiencing a prevalence of 17.1% compared with 5.6% in men. The cumulative incidence was 43% in women and 18% in men.1,5 Another study, utilizing National Health Interview Survey data, reported a prevalence of 13.2% in the general population, with rates of 17.5% in women and 8.6% in men.6 In a Korean nationwide cross-sectional survey, structured interviews with 1,507 subjects conducted by trained interviewers showed a 1-year migraine prevalence of 6.1% in the overall population, 9.2% in women, and 2.9% in men.7 These findings consistently show that migraines are more common in women than in men.
Sex differences in migraine prevalence vary across age groups. Studies of pediatric migraines reported a prevalence of 4%, with no sex differences before puberty, and an increase in women older than 11 years.8,9 The migraine prevalence was 4.0% in men and 6.4% in women aged between 12 and 17 years and it was highest among those 30–39 years of age for both sexes (24.4% in women; 7.4% in men) and lowest after the age of 60 years (5% in women; 1.6% in men). The difference in migraine prevalence between sexes was highest in the 30–39 age group.1,5 Previous study revealed a bimodal peak in 1-year migraine prevalence at 20 and 50 years for both sexes. After the age of 10 years, migraine prevalence was consistently higher in women than in men. The difference in prevalence was greatest at age 30.2 years, with a 2.9-fold difference, and remained about 2-fold after the age of 42 years.6
Women with migraines tend to experience migraines for longer durations, at greater frequency, and in closer association with photophobia, phonophobia, nausea, and vomiting, compared with men.10-13 The impact of sex on migraines becomes more significant after the age of 30 in women.10 Although there is no significant difference in migraine medication use according to sex, women with migraines are more likely than men to take both acute and preventive medications.14 Prognostically, a previous study reported that 23% of pediatric migraines resolve before the age of 25, with a higher resolution rate in men (34%) than in women (15%). By the age of 50 year, 46% were migraine-free, with no significant sex difference.8
ROLES OF SEX HORMONES IN MIGRAINE
Sex differences in migraine prevalence emerge at puberty when women begin to menstruate and experience periodic hormonal changes during the menstrual cycle. Estrogen and progesterone are known to be related to migraine pathophysiology. Gonadotropin-releasing hormone (GnRH) from the hypothalamus stimulates the secretion of luteinizing hormone (LH) and follicular stimulating hormone (FSH) from the pituitary. LH and FSH trigger ovarian maturation and ovulation, promoting the secretion of estrogen and progesterone, which are regulated by feedback mechanisms.15 Sex hormones have low molecular weights and lipophilic properties, allowing them to passively diffuse across the blood-brain barrier. Consequently, central and peripheral levels of sexual steroid hormones are comparable, exerting an influence on migraines through neurotransmitter and pain modulation systems.16,17 However, the effects of sexual steroid hormones on migraines are complex, with mixed effects reported depending on the site of action, duration, and concentration.
1. The impact of sex hormones on the neurotransmitter system
Several neurotransmitter systems, including serotonin (5-hydroxytryptamine [5-HT]), noradrenaline, gamma-aminobutyric acid (GABA), opioids, and glutamate have been implicated in the pathophysiology of migraines. Sexual steroid hormones play a crucial role in the regulation of these neurotransmitter systems.
Serotonin is a neurotransmitter associated with migraines, and an increased serotonin synthesis capacity has been observed in migraine patients.17 Triptans, which are commonly used to treat migraines, are selective serotonin receptor agonists (5-HT1B, 5-HT1D). Tryptophan hydroxylase (TPH) is the rate-limiting enzyme for serotonin synthesis, and a previous study on oophorectomized monkeys reported that TPH mRNA activity increased nine-fold with estrogen supplementation and five-fold with an estrogen-progesterone combination.18 The serotonin reuptake transporter (SERT) at the presynaptic area is responsible for serotonin reuptake and degradation. In oophorectomized monkeys, short-term estrogen supplementation (<28 days) decreased SERT mRNA activity.19,20 Monoamine oxidase (MAO) is the primary enzyme that degrades serotonin. Estrogen decreases the expression of MAO genes in the hypothalamus and dorsal raphe nucleus.21 These findings suggest that estrogen increases serotonergic activity and potentially promotes migraine symptoms. Progesterone may have fewer influences on the serotonin system, and the serotonergic system may respond differently based on the duration of estrogen supplementation.
The noradrenergic system plays a crucial role in modulating the signal-to-noise ratio of neurons in response to incoming afferent stimuli. Estrogen is known to increase the gene expression of tyrosine hydroxylase, the rate-limiting enzyme in noradrenaline biosynthesis.22 In animal studies, estrogen infusion elevated noradrenaline secretion in the mediobasal hypothalamus and enhanced the gene expression of tyrosine hydroxylase in the locus coeruleus.23 Glutamic acid, an excitatory neurotransmitter linked to pain transmission, is also influenced by estrogen. Acute exposure to estrogen increases the glutaminergic neuronal firing of hippocampal Purkinje cells, while chronic exposure enhances dendritic spine density and excitatory synapses of hippocampal glutaminergic neurons.17
Glutamic acid decarboxylase is responsible for converting glutamate to GABA, an inhibitory neurotransmitter, and shows increased activity with estrogen. This results in heightened GABA release and up-regulation of GABA receptors.24 Estrogen injections can also increase enkephalin mRNA activity in the spinal cords of female rats. Both estrogen and progesterone contribute to an increased binding affinity of kappa-opioid receptors in oophorectomized rats.25-27 These findings emphasize the intricate influence of sexual steroid hormones on various neurotransmitter systems and their potential role in modulating neuronal responses and pain pathways.
2. Calcitonin gene-related peptide and sex differences in migraines
Calcitonin gene-related peptide (CGRP) is a key pathophysiological factor in migraines. It is released in the brain and influences pain transmission within the trigeminal nervous system. During a migraine attack, CGRP levels increase jugular blood flow, which subsequently decrease after migraine symptoms improve in response to administration of sumatriptan.28,29 An intravenous CGRP injection can trigger migraine attacks in 65% of migraine patients but not in control groups, implying that the former may possess a lower threshold to CGRP.30-32 Clinically, CGRP receptor antagonist and monoclonal antibodies against CGRP are employed in migraine treatment.
Several studies suggest a potential association between CGRP and sex differences in migraines. The administration of dural CGRP induces a decrease in the facial withdrawal threshold to mechanical stimuli in female rats but not in male rats. Intracisternal injections of brain-derived neurotrophic factor, and interleukin-6 decrease the facial withdrawal threshold in rats, which recover after 72 hours, and subsequent dural CGRP administration delays the recovery response only in female rats.33 In both sexes, administration of CGRP to the trigeminal ganglion results in a decrease in the threshold of mechanical stimuli, but recovery of mechanical allodynia with minocycline and propentofylline was observed only in male rats.34 CGRP induces vasodilation of meningeal vessels, a process linked to migraine pathophysiology.35 A study investigating vasodilation responses in ovariectomized rats found that estradiol premedication increased vasodilation in responses to electrical stimulation. In meningeal arterial system, estradiol increases the CGRP-immunoreactive sensory innervation and promotes release of CGRP from nerve endings innervating the meningeal artery.36
Transient receptor potential ankyrin 1 (TRPA1), and transient receptor potential melastatin 8 (TRPM8), both of which are transient receptor potential channels of trigeminal neurons, are closely related to migraine pathophysiology. TRPA1, found on the primary afferent neurons innervating the meninges, releases CGRP when activated. Nitroglycerin increases TRPA1-mediated neuronal activity, promoting nociceptive hypersensitivity. TRPM8 attenuates migraine symptom and TRPA1-related pain. The antinociceptive action of TRPM8 is more pronounced in male rats and necessitates testosterone. Exogenous testosterone induces the recovery of mechanical hypersensitivity in female and orchiectomized male rats, implying potential sex-dependent effects of TRPM8 on migraines.37
Interplay between prolactin and CGRP may contribute to sex differences in migraines. Although serum prolactin levels do not differ between migraineurs and controls, prolactin increases during a migraine attack but not in tension-type headaches.38,39 Dural prolactin administration decreases the withdrawal threshold for mechanical stimuli in female rats but not in male rats. An immunohistochemical analysis reveals a prolactin receptor in the dural afferent nerve of female rats but not in male rats. Prolactin stimulates the release of CGRP and activates the dural trigeminal ganglion neurons only in female rats.40
MENSTRUATION AND MIGRAINES
1. Menarche, menstruation, and hormonal dynamics
Menarche marks the onset of the first menstrual period in women, and in Korea, the mean age of menarche is reportedly 12.9±1.18 years.41 Following menarche, approximately 10 anovulatory menstrual cycles occur per year, with a serum estrogen level ranging from 10 to 156 pg/mL. Over subsequent years, the serum estrogen level gradually aligns with that of an adult.42 This transition period signifies the maturation of the reproductive system and involve adjustments in hormonal levels and menstrual cycle characteristics.
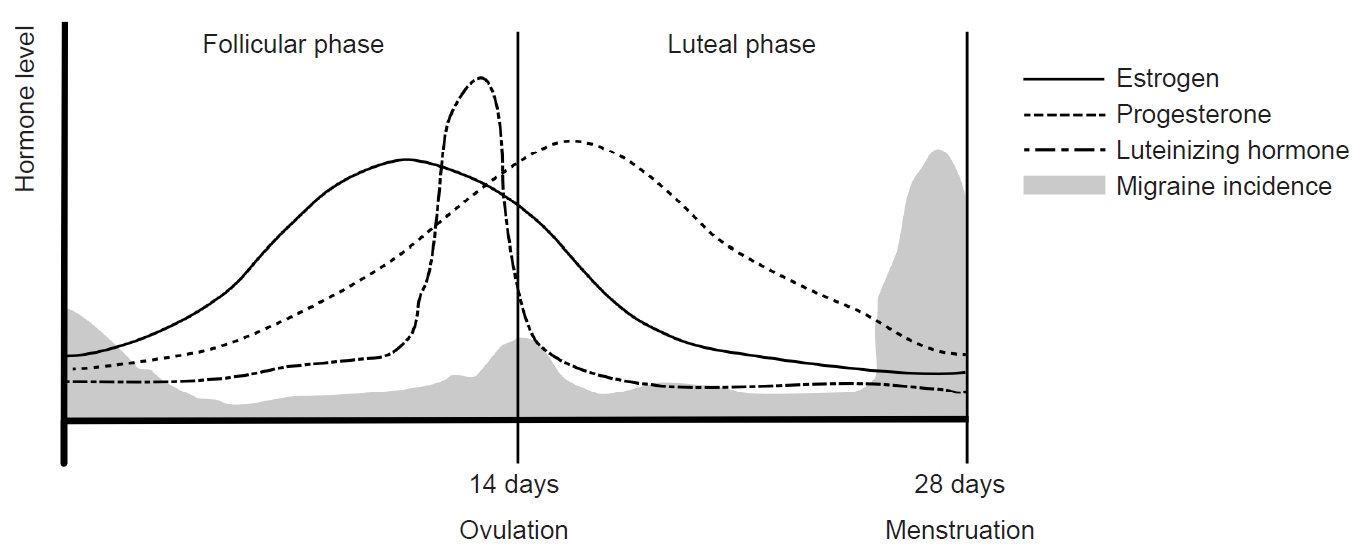
The menstrual cycle can be divided into two main phases: the follicular phase and the luteal phase, as determined by the occurrence of ovulation. The follicular phase spans from the onset of menstrual bleeding to just before ovulation, while the luteal phase extends from ovulation to the commencement of the next menstrual cycle. With an average duration of 28 days, a menstrual cycle is characterized by a constant luteal period of 14 days, and the entire cycle is predominantly influenced by the follicular phase. In the follicular phase, FSH and LH stimulate follicle maturation and estrogen secretion, respectively, in the ovary. The early follicular period sees low serum estrogen levels ranging from 25 to 50 pg/mL, with progesterone levels remaining below 1 ng/mL. As the follicular phase progresses, estrogen levels rise steadily, reaching a peak of 100 to 400 pg/mL during the late follicular to early luteal periods. This estrogen surge triggers the abrupt secretion of LH, leading to ovulation.
In the subsequent luteal phase, the corpus luteum, a temporary endocrine structure formed after ovulation, produces both estrogen and progesterone. During this phase, estrogen inhibits GnRH, FSH, and LH, while progesterone plays a crucial role in maintaining the endometrium of the uterus. In the absence of fertilization, serum estrogen levels remain between 200 and 300 pg/mL throughout the luteal period, decreasing to 25–50 pg/mL before the onset of the next menstrual cycle. Concurrently, serum progesterone levels increase to 6–10 ng/mL during the mid-luteal period and decrease to 2 ng/mL in the late luteal period. These intricate hormonal dynamics orchestrate the menstrual cycle, regulating the physiological processes essential for reproductive health (Figure 2).
2. Menstrual migraine and estrogen withdrawal theory
A menstrual migraine is defined as a migraine that occurs from two days before to three days after the onset of menstruation. Previous studies have reported that menstrual migraines often peak 2 days before menstruation and are not associated with ovulation.43,44 Additionally, there is a reported connection between serum estrogen levels and menstrual migraines. These migraines frequently occur during the late luteal and early follicular periods when serum estrogen levels decrease rapidly. In contrast, migraine attacks are less frequent when serum estrogen levels are stable.45 For individuals with migraines, attacks during the perimenstrual period tend to be more severe and last longer compared with other phases of the menstrual cycle.46,47 Although the mean serum estrogen level does not differ significantly between migraineurs and those without migraines, migraineurs exhibit a more rapid decline in estrogen during the luteal period compared with controls.48
These findings support a close relationship between estrogen decline and migraines, which underlies the estrogen withdrawal theory. In the late luteal period, estrogen supplementation has been shown to delay migraine attacks, while progesterone does not exert the same effect.49,50 However, during the mid-follicular phase, migraines reportedly did not occur when short-acting estrogen was administered to induce a change in estrogen concentration. This suggests that priming of estrogen may be necessary before actual estrogen withdrawal triggers migraines.51
3. Difference in menstrual migraine between migraines with and without aura
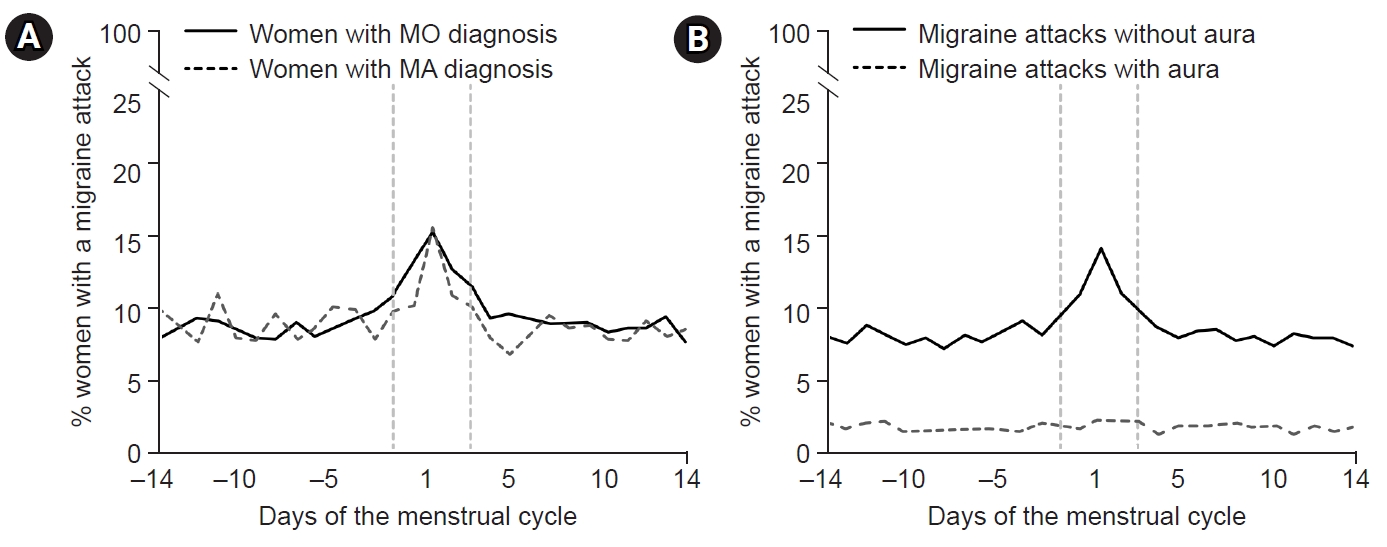
An interesting distinction in menstrual migraine has been observed between individuals with migraine without aura (MO) and those with migraine with aura (MA). In a study examining headache patterns during the menstrual cycle in 81 women with migraines, menstrual cycles were divided into five study periods (3–7 days before menses, 1–2 days before menses, 0–1 days after menses, 3–5 days after menses, and 14–15 days before menses) and a control period. The study found that the risk of MO increased in the period 1–2 days before menses (odds ratio [OR], 2.04; 95% confidence interval [CI], 1.49–2.81), and the period 0–1 day after menses (OR, 1.80; 95% CI, 1.40–2.30). However, the risk of MA did not differ significantly among study periods.52 Another study investigated the differences in migraine symptoms between perimenstrual and non-perimenstrual migraines in women with migraines. Perimenstrual migraine headaches were found to have a longer duration, higher recurrence risk, increased triptan intake, higher headache intensity, and more pronounced photophobia and phonophobia compared with non-perimenstrual headaches. However, the prevalence of aura was lower in perimenstrual headaches (OR, 0.8; 95% CI, 0.6–1.0).46 A further investigation into perimenstrual migraine differences between women diagnosed with MO and MA revealed that, while the prevalence of migraine attacks did not differ between the two groups (59% for MO versus 53% for MA, p=0.176). The risk of migraine attack without aura was significantly higher during the perimenstrual period in both MO (OR, 1.53; 95% CI, 1.44–1.62) and MA groups (OR, 1.53; 95% CI, 1.44–1.62). However, the risk of MA attacks did not show a significant change within the perimenstrual period (OR, 1.08; 95% CI, 0.93–1.26). This implies that the most common form of perimenstrual migraine is MO, even in individuals diagnosed with MA (Figure 3).53 The cause of these differences is unclear but may be related to variations in the effects of estrogen on the trigeminal-vascular system and cortical spreading depression (CSD). In migraine patients, headache occurrence is related to the trigemino-vascular system and is caused by the short-term effects of estrogen. In CSD, the occurrence of aura is thought to be related to the long-term effects of estrogen.54 An animal study investigating the association between estrogen and CSD found that CSD was more frequently observed in female rats than in male and ovariectomized female rats. In female rats, the risk of CSD remained unchanged during normal menstrual cycles and with long-term (3-week) estrogen supplementation. However, when the rats underwent 2 weeks of estrogen treatment followed by 1 week of withdrawal, the incidence of CSD increased.55 This study suggests that the development of CSD is not directly linked to estrogen withdrawal during the natural menstrual cycle. Instead, it appears to be associated with withdrawal after a prolonged period of estrogen exposure.
PREGNANCY, LACTATION, AND MIGRAINES
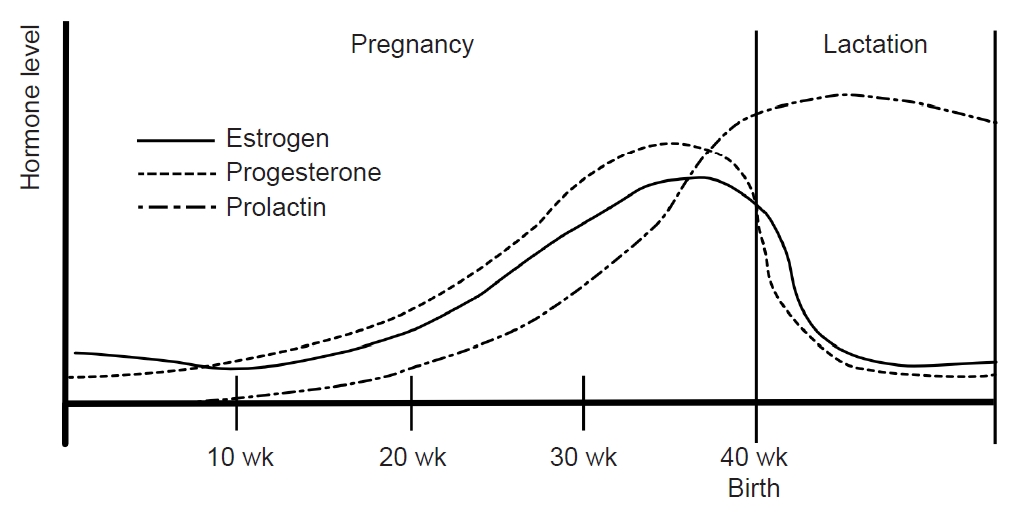
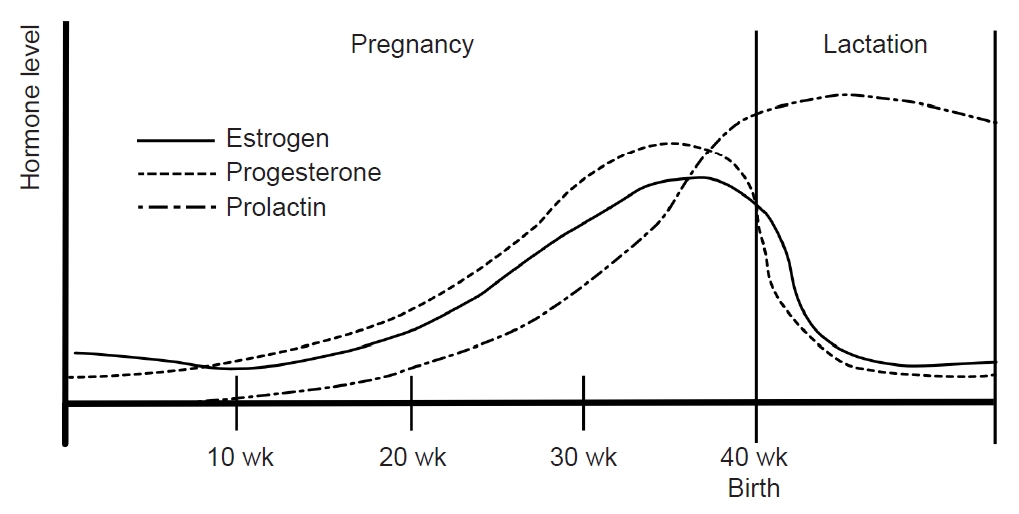
During pregnancy, the consistent production of estrogen and progesterone by the placenta results in a stable hormonal environment free from the fluctuations of the menstrual cycle. In the third trimester, levels of serum estrogen (30–40 times) and progesterone (20 times) are significantly elevated compared with the peak levels in the menstrual cycle.56 Following childbirth, the hormonal status and menstrual cycles vary depending on whether lactation occurs. Prolactin, a pituitary hormone associated with lactation, maintains a serum level of 10–25 ng/mL before pregnancy. During pregnancy, estrogen and progesterone promote mammary gland maturation while inhibiting prolactin secretion and lactation. Within 24 hours postpartum, serum estrogen and progesterone decrease, and prolactin increases, initiating lactation (Figure 4).57
Pregnant women with a history of migraines reportedly show significant improvement in the second and third trimesters, with improvement rates of 46.8% in the first trimester, 83% in the second trimester, and 87% in the third trimester. Some women even experience complete remission of migraines during pregnancy, with remission rates of 11% in the first trimester, 53% in the second trimester, and 73% in the third trimester. Notably, many of these changes in migraine patterns during pregnancy revert to their previous state after childbirth.58
These changes in migraines during pregnancy are likely associated with hormonal influences. The continuous high and steady serum estrogen levels during pregnancy align with the estrogen withdrawal theory, which posits that stable estrogen levels lead to migraine improvement. The reversion of migraine patterns after childbirth supports this hypothesis.59-61 Another potential mechanism involves the influence of pregnancy on pain transmission. Animal studies have shown that both the nociceptive threshold and dynorphin increase in the lumbar spinal cords of pregnant rats. Simulated pregnancy in animal models using estrogen and progesterone demonstrated similar opioid analgesia in the spinal cord.27
However, some women without a previous history of migraines experience new-onset migraines during pregnancy, with occurrence rates of 1.3% to 18%. This phenomenon is predominantly associated with MA. It is more prevalent during the first trimester and is believed to be related to a rapid increase in estrogen levels rather than estrogen withdrawal.58,59
During lactation, mechanical breast stimulation enhances prolactin production. Prolactin inhibits the release of GnRH, suppressing estrogen and progesterone release and preventing ovulation.57 Subsequently, serum estrogen levels remain consistently low without fluctuations in lactating women (Figure 4). Clinical studies have found that most women who bottle-feed their children experience migraine recurrence within 1 month after delivery compared with only 43.2% of breastfeeding women.58 In women who bottle-feed, the decrease in prolactin restarts the menstrual cycle, leading to hormonal fluctuations that can increase the frequency of migraines.57
MENOPAUSE AND MIGRAINES
Menopause is the cessation of menstruation for more than 1 year without other identifiable causes. The mean age of menopause is approximately 50 years, with a reported mean menopausal age in Korea of 49.3±3.5 years.62 Distinct effects on migraines have been observed between menopause and the perimenopausal period.
A woman’s ovarian follicles are limited in number, with most producing approximately 400–500 ova over 40 years, after which ovarian function diminishes. Women typically experience an irregular menstrual cycle for 2–8 years before entering menopause, a stage known as perimenopause and characterized by a decline in ovarian function. During this phase, women experience hypergonadotropism, hypoestrogenism, hypoprogesteronism, and greater estrogen fluctuations.63 The excessive stimulation of remaining follicles results in severe estrogen fluctuations. After menopause, serum estrogen and progesterone levels remain consistently low without fluctuation.64 According to the estrogen withdrawal hypothesis, estrogen fluctuations during perimenopause can contribute to frequent migraine attacks, with an anticipated improvement in migraines after menopause.
A cross-sectional study reported an increase in migraine prevalence during perimenopause, followed by a decrease after spontaneous menopause. However, this study focused on women aged 40 to 54 years and did not comprehensively analyze postmenopausal women.65 Another study indicated an increased risk of migraines in perimenopausal woman (OR, 1.42; 95% CI, 1.03–1.94) but not significantly in postmenopausal women (OR, 1.27; 95% CI, 0.83–1.92) compared with premenopausal women.66 These findings support the exacerbation of migraines during the perimenopausal period, although the effect of menopause on migraines remains inconclusive.
While some studies have reported a decrease in migraine prevalence after menopause, others found no significant differences among premenopausal, perimenopausal, and postmenopausal periods.67-69 In women with migraines, improvement after menopause ranges from 8%–36%, worsening from 9%–42%, and remaining unchanged from 27%–64%. Additionally, 8%–13% of women may experience their first migraine after menopause.15 Other clinical factors may affect postmenopausal migraines. The risk of migraines risk in cases of early artificial menopause, such as those associated with a hysterectomy or oophorectomy, with a higher risk has been reported in surgical menopause compared with spontaneous menopause. Previous studies reported a migraine risk of 27% in surgical menopause but 7% in spontaneous menopausal women.64,65,70 Previously, we reported that a shorter lifetime number of year of menstruation (LNYM) and hormone replacement therapy (HRT) may contribute to an increased risk of migraines in postmenopausal women.71 The LNYM represents the cumulative duration of menstruation cycles and cumulative exposure to endogenous hormones over a women’s life.72,73 Our study suggests an elevated risk of postmenopausal migraine in women with either exogenous hormone exposure or insufficient endogenous hormone exposure.
The long-term incidence of migraines after menopause has not been fully elucidated. In a previous study utilizing the Korean National Health Insurance Service database, we reported that the risk of migraines was higher in women in longer postmenopausal groups, including those postmenopausal for ≥15 years (HR, 1.196; 95% CI, 1.169–1.224), <15 years (HR, 1.09; 95% CI, 1.069–1.111), and <10 years (HR, 1.042; 95% CI, 1.027–1.058) compared with the <5 years group. Our results suggest that an extended period after menopause may increase the risk of migraines again.71 The mechanism is unclear, but it is possible that prolonged low estrogen levels may lower pain thresholds due to decreased GABAergic and opioidergic effects of estrogen.
EXOGENOUS HORMONAL FACTOR (CONTRACEPTION AND HORMONE REPLACEMENT THERAPY) AND MIGRAINES
In women, physiological processes such as menstruation, pregnancy, and lactation represent endogenous hormonal changes, while contraception and HRT introduce exogenous hormonal alterations. Male-to-female transsexuals taking estrogen often experience heightened and exacerbated headaches.74 Conversely, some prior studies have indicated that continuous estrogen or HRT may alleviate headache symptoms, suggesting a potential influence of exogenous estrogen on migraine.60,75 The impact of exogenous hormones on migraines can vary based on type, composition, timing, and route of administration.
Contraception is commonly utilized to manage menstrual cycles and prevent pregnancy, with various administration methods available. This discussion focuses on combined oral contraceptives (COCs), the most frequently used form. The notion that COCs increase the prevalence of migraines and worsen migraine symptoms is generally accepted.15 Approximately 70% of women who experience migraines report exacerbation of symptoms with COCs.76 This exacerbation tends to be more pronounced in women with MA compared with those with MO, and the frequency of auras often increases.60 These exacerbations are frequently observed during pill-free periods, with a four-fold increase in the prevalence of migraine attacks on pill-free days 3–6.77
Among women without a history of migraine, COC use has been associated with a significant increase in migraine prevalence (OR, 1.4; 95% CI, 1.2–1.7) compared with those who have never used COCs.78 However, conflicting findings exist, with other studies reporting that migraine headache patterns remained unchanged in 44%–67%, worsened in 24%–36%, and improved in 5%–8% with the use of COCs.79-81 Another study suggested that continuous estrogen administration could decrease the symptoms and frequency of migraines.60 These discrepancies in the effects of COCs on migraines can be attributed to differences in the composition and dosage of COCs, with recent research suggesting that low-dose estrogen pills have a reduced impact on migraines.15
After menopause, women experience various postmenopausal symptoms, such as hot flushes, irritability, insomnia, mood changes, and osteoporosis. HRT is commonly used to alleviate menopausal symptoms and may involve estrogen alone or in combination with progesterone.15 The effects of HRT on migraines remain inconsistent across studies. In one study investigating changes in migraine symptoms during HRT, migraine symptoms worsened in 21%, improved in 22%–23%, and remained unchanged in 57% of migraineurs.82 Another study reported that migraine symptoms did not change in 77% of migraineurs with HRT.80 In a population-based study, headache prevalence increased in women who did not have a history of headaches.75 Another population-based study of 17,107 postmenopausal women reported that current HRTs significantly increase the risk of migraine (OR, 1.42; 95% CI, 1.24–1.62) compared with a non-HRT population.83
Differences in HRT composition and administration methods can influence its effects on migraines. Continuous estrogen administration is associated with a decrease in migraine severity and attack frequency.60 Non-oral estrogen may have a more favorable impact on migraines compared with oral administration.84 For example, in one study, users of transdermal estradiol patches did not experience changes in headache symptoms, while headaches worsened in those receiving oral HRT.85 However, a population-based cross-sectional study reported that the routes of HRT administration were not associated with migraine risk, while an HRT group had a greater migraine risk compared with the non-HRT group.86
In summary, COCs are generally associated with migraine exacerbations, and low-estrogen pills may have a reduced impact on migraines. HRT also tends to exacerbate migraines, but the effects are influenced by composition, dose, and route of administration.
CONCLUSION
Women’s migraines are profoundly influenced by hormonal changes, and the dynamic nature of hormonal fluctuations throughout their lives contributes to the complexity of this relationship. The same hormone can elicit opposite effects on migraines depending on the timing, dose, and other factors. It is therefore important to understand the fluctuations in female hormones and how they affect migraines at different times. In addition to exploring previous neurotransmitter systems, recent studies investigated CGRP as a potential mechanism underlying hormonal effects on migraines. These studies contribute valuable insights into the intricate interplay between hormonal dynamics and the manifestation of migraines. Understanding the effects of hormones on migraines is a crucial aspect of research into the mechanisms of migraines and their treatment.
Availability of data and material
Not applicable.
Author contributions
Conceptualization: JWP; Data curation: SK; Investigation: SK; Writing–original draft: SK; Writing–review and editing: JWP.
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding statement
Not applicable.
Acknowledgements
Not applicable.
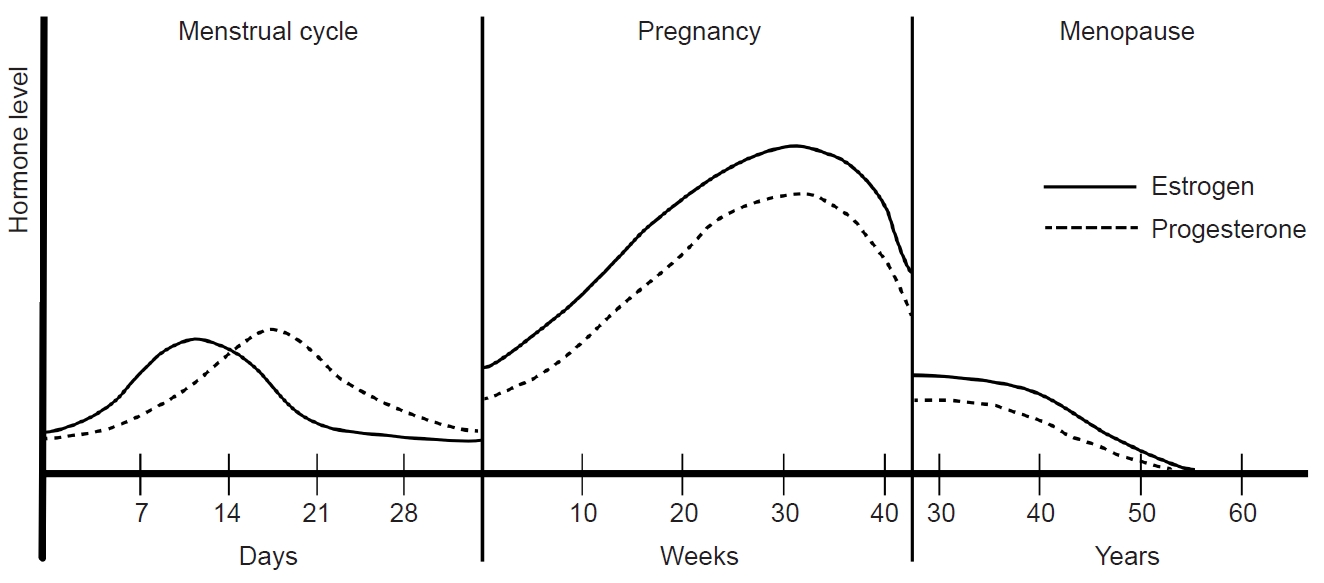
Figure 1.A fluctuations in women’s sex hormones across life stages. Women experience fluctuations in sex hormones with reproductive events throughout their lives. After menarche, cyclical fluctuations in estrogen and progesterone occur with menstruation. During pregnancy, estrogen and progesterone remain elevated, normalize after childbirth, and decrease during menopause.
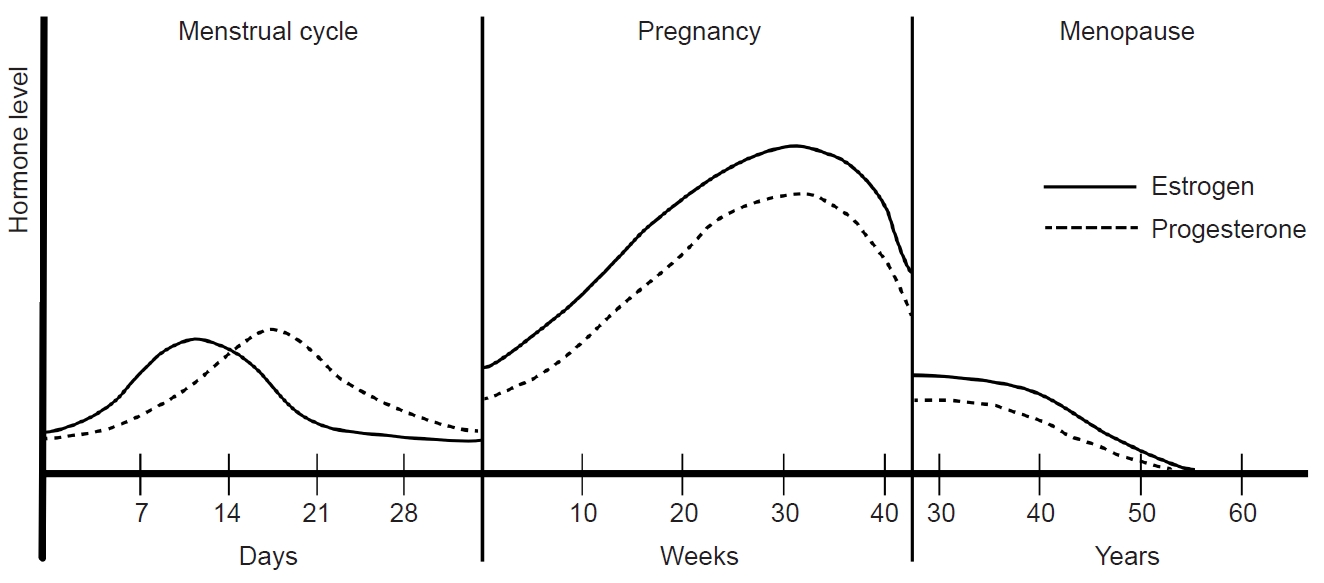
Figure 2.Hormonal changes and menstrual migraine during the menstrual cycle: The menstrual cycle can be divided into follicular and luteal phases according to ovulation. Following ovulation, the corpus luteum produces estrogen and progesterone. In the absence of fertilization, estrogen secretion decreases, leading to the initiation of menstruation. Menstrual migraines occur during the period from 2 days before to 3 days after menstruation.
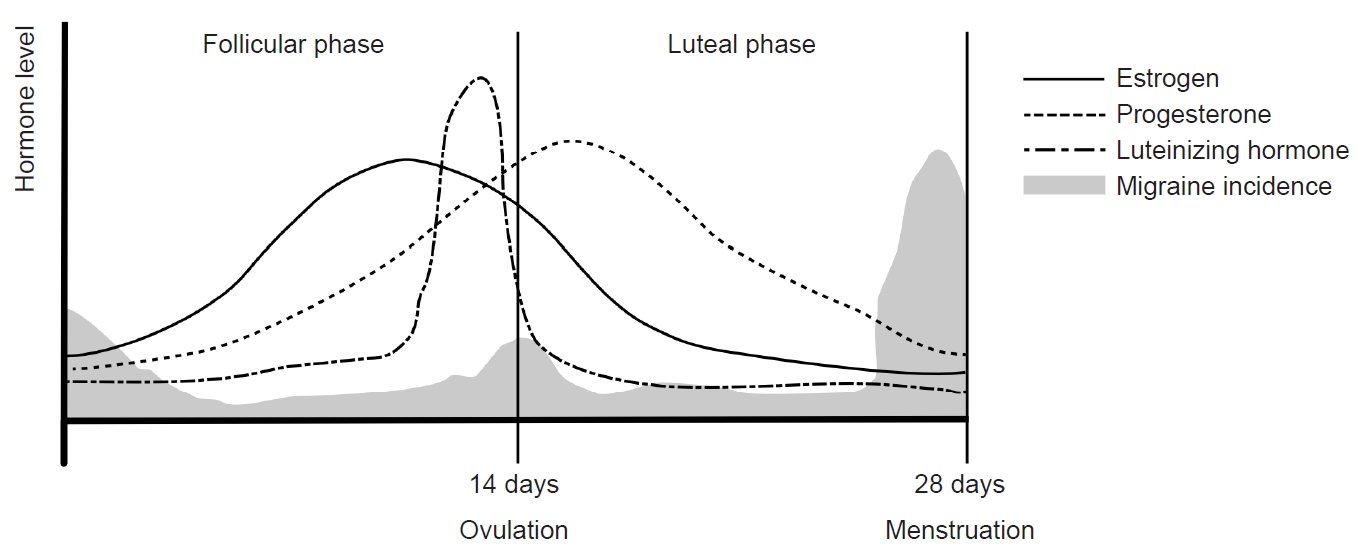
Figure 3.Differences in perimenstrual migraines between women diagnosed with migraines without aura (MO) and migraines with aura (MA): (A) The prevalence of migraine attacks did not differ between women with MO and MA. (B) The risk of MO was significantly higher during the perimenstrual period in all groups of women with migraines. Modified from Verhagen et al. (Cephalalgia 2023;43:3331024231164322).53
Figure 4.Sex hormones during pregnancy and lactation: During pregnancy, estrogen and progesterone remain at high levels without fluctuating. Immediately after childbirth, estrogen and progesterone decrease and prolactin increases. During lactation, prolactin is maintained at a high concentration and inhibits the secretion of estrogen and progesterone. Their concentrations remain low without fluctuations.
REFERENCES
- 1. Stewart WF, Wood C, Reed ML, Roy J, Lipton RB; AMPP Advisory Group. Cumulative lifetime migraine incidence in women and men. Cephalalgia 2008;28:1170-1178.ArticlePubMedPDF
- 2. Broner SW, Bobker S, Klebanoff L. Migraine in women. Semin Neurol 2017;37:601-610.ArticlePubMed
- 3. Pavlovic JM, Akcali D, Bolay H, Bernstein C, Maleki N. Sex-related influences in migraine. J Neurosci Res 2017;95:587-593.ArticlePubMedPDF
- 4. Allais G, Chiarle G, Sinigaglia S, Airola G, Schiapparelli P, Benedetto C. Estrogen, migraine, and vascular risk. Neurol Sci 2018;39:11-20.ArticlePubMedPDF
- 5. Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68:343-349.ArticlePubMed
- 6. Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia 2010;30:1065-1072.ArticlePubMedPDF
- 7. Kim BK, Chu MK, Lee TG, Kim JM, Chung CS, Lee KS. Prevalence and impact of migraine and tension-type headache in Korea. J Clin Neurol 2012;8:204-211.ArticlePubMedPMC
- 8. Bille B. A 40-year follow-up of school children with migraine. Cephalalgia 1997;17:488-491.ArticlePubMedPDF
- 9. Bille BS. Migraine in school children. A study of the incidence and short-term prognosis, and a clinical, psychological and electroencephalographic comparison between children with migraine and matched controls. Acta Paediatr Suppl (Upps) 1962;136:1-151.PubMed
- 10. Bolay H, Ozge A, Saginc P, et al. Gender influences headache characteristics with increasing age in migraine patients. Cephalalgia 2015;35:792-800.ArticlePubMedPDF
- 11. Kallela M, Wessman M, Färkkilä M, et al. Clinical characteristics of migraine in a population-based twin sample: similarities and differences between migraine with and without aura. Cephalalgia 1999;19(3):151-158.ArticlePubMedPDF
- 12. Wöber-Bingöl C, Wöber C, Karwautz A, et al. Clinical features of migraine: a cross-sectional study in patients aged three to sixty-nine. Cephalalgia 2004;24:12-17.ArticlePubMedPDF
- 13. Kelman L. Pain characteristics of the acute migraine attack. Headache 2006;46:942-953.ArticlePubMed
- 14. Buse DC, Loder EW, Gorman JA, et al. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache 2013;53:1278-1299.ArticlePubMed
- 15. Martin VT, Behbehani M. Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis: part 2. Headache 2006;46:365-386.ArticlePubMed
- 16. Aloisi AM. Gonadal hormones and sex differences in pain reactivity. Clin J Pain 2003;19:168-174.ArticlePubMed
- 17. Martin VT, Behbehani M. Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis: part I. Headache 2006;46:3-23.ArticlePubMed
- 18. Pecins-Thompson M, Brown NA, Kohama SG, Bethea CL. Ovarian steroid regulation of tryptophan hydroxylase mRNA expression in rhesus macaques. J Neurosci 1996;16:7021-7029.ArticlePubMedPMC
- 19. Pecins-Thompson M, Brown NA, Bethea CL. Regulation of serotonin re-uptake transporter mRNA expression by ovarian steroids in rhesus macaques. Brain Res Mol Brain Res 1998;53:120-129.ArticlePubMed
- 20. Smith LJ, Henderson JA, Abell CW, Bethea CL. Effects of ovarian steroids and raloxifene on proteins that synthesize, transport, and degrade serotonin in the raphe region of macaques. Neuropsychopharmacology 2004;29:2035-2045.ArticlePubMedPDF
- 21. Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol 2002;23:41-100.ArticlePubMed
- 22. Herbison AE, Simonian SX, Thanky NR, Bicknell RJ. Oestrogen modulation of noradrenaline neurotransmission. Novartis Found Symp 2000;230:74-93.ArticlePubMedPDF
- 23. Pau KY, Hess DL, Kohama S, Bao J, Pau CY, Spies HG. Oestrogen upregulates noradrenaline release in the mediobasal hypothalamus and tyrosine hydroxylase gene expression in the brainstem of ovariectomized rhesus macaques. J Neuroendocrinol 2000;12:899-909.ArticlePubMed
- 24. Shughrue PJ, Merchenthaler I. Estrogen is more than just a “sex hormone”: novel sites for estrogen action in the hippocampus and cerebral cortex. Front Neuroendocrinol 2000;21:95-101.ArticlePubMed
- 25. Amandusson A, Hallbeck M, Hallbeck AL, Hermanson O, Blomqvist A. Estrogen-induced alterations of spinal cord enkephalin gene expression. Pain 1999;83:243-248.ArticlePubMed
- 26. Gordon FT, Soliman MR. The effects of estradiol and progesterone on pain sensitivity and brain opioid receptors in ovariectomized rats. Horm Behav 1996;30:244-250.ArticlePubMed
- 27. Medina VM, Dawson-Basoa ME, Gintzler AR. 17 beta-estradiol and progesterone positively modulate spinal cord dynorphin: relevance to the analgesia of pregnancy. Neuroendocrinology 1993;58:310-315.ArticlePubMedPDF
- 28. Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol 1993;33:48-56.ArticlePubMed
- 29. Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 1990;28:183-187.ArticlePubMed
- 30. Petersen KA, Lassen LH, Birk S, Lesko L, Olesen J. BIBN4096BS antagonizes human alpha-calcitonin gene related peptide-induced headache and extracerebral artery dilatation. Clin Pharmacol Ther 2005;77:202-213.ArticlePubMed
- 31. Hansen JM, Hauge AW, Olesen J, Ashina M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia 2010;30:1179-1186.ArticlePubMedPDF
- 32. Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia 2002;22:54-61.ArticlePubMedPDF
- 33. Avona A, Burgos-Vega C, Burton MD, Akopian AN, Price TJ, Dussor G. Dural calcitonin gene-related peptide produces female-specific responses in rodent migraine models. J Neurosci 2019;39:4323-4331.ArticlePubMedPMC
- 34. Araya EI, Turnes JM, Barroso AR, Chichorro JG. Contribution of intraganglionic CGRP to migraine-like responses in male and female rats. Cephalalgia 2020;40:689-700.ArticlePubMedPDF
- 35. Russo AF, Hay DL. CGRP physiology, pharmacology, and therapeutic targets: migraine and beyond. Physiol Rev 2023;103:1565-1644.ArticlePubMed
- 36. Gupta S, Villalón CM, Mehrotra S, et al. Female sex hormones and rat dural vasodilatation to CGRP, periarterial electrical stimulation and capsaicin. Headache 2007;47:225-235.ArticlePubMed
- 37. Alarcón-Alarcón D, Cabañero D, de Andrés-López J, et al. TRPM8 contributes to sex dimorphism by promoting recovery of normal sensitivity in a mouse model of chronic migraine. Nat Commun 2022;13:6304.ArticlePubMedPMC
- 38. Bosco D, Belfiore A, Fava A, et al. Relationship between high prolactin levels and migraine attacks in patients with microprolactinoma. J Headache Pain 2008;9:103-107.ArticlePubMedPMCPDF
- 39. Guldiken S, Guldiken B, Demir M, et al. Soluble CD40 ligand and prolactin levels in migraine patients during interictal period. J Headache Pain 2011;12:355-360.ArticlePubMedPMCPDF
- 40. Avona A, Mason BN, Burgos-Vega C, et al. Meningeal CGRP-prolactin interaction evokes female-specific migraine behavior. Ann Neurol 2021;89:1129-1144.ArticlePubMedPMC
- 41. Seo MY, Kim SH, Juul A, Park MJ. Trend of menarcheal age among Korean girls. J Korean Med Sci 2020;35:e406.ArticlePubMedPMCPDF
- 42. Jenner MR, Kelch RP, Kaplan SL, Grümbach MM. Hormonal changes in puberty. IV. Plasma estradiol, LH, and FSH in prepubertal children, pubertal females, and in precocious puberty, premature thelarche, hypogonadism, and in a child with a feminizing ovarian tumor. J Clin Endocrinol Metab 1972;34:521-530.ArticlePubMed
- 43. MacGregor EA, Hackshaw A. Prevalence of migraine on each day of the natural menstrual cycle. Neurology 2004;63:351-353.ArticlePubMed
- 44. Dalton K. Progesterone suppositories and pessaries in the treatment of menstrual migraine. Headache 1973;12:151-159.ArticlePubMed
- 45. MacGregor EA, Frith A, Ellis J, Aspinall L, Hackshaw A. Incidence of migraine relative to menstrual cycle phases of rising and falling estrogen. Neurology 2006;67:2154-2158.ArticlePubMed
- 46. van Casteren DS, Verhagen IE, van der Arend BWH, van Zwet EW, MaassenVanDenBrink A, Terwindt GM. Comparing perimenstrual and nonperimenstrual migraine attacks using an e-diary. Neurology 2021;97:e1661-e1671.ArticlePubMedPMC
- 47. Vetvik KG, Benth JŠ, MacGregor EA, Lundqvist C, Russell MB. Menstrual versus non-menstrual attacks of migraine without aura in women with and without menstrual migraine. Cephalalgia 2015;35:1261-1268.ArticlePubMedPDF
- 48. Pavlović JM, Allshouse AA, Santoro NF, et al. Sex hormones in women with and without migraine: evidence of migraine-specific hormone profiles. Neurology 2016;87:49-56.ArticlePubMedPMC
- 49. Somerville BW. The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology 1972;22:355-365.ArticlePubMed
- 50. Somerville BW. Estrogen-withdrawal migraine. II. Attempted prophylaxis by continuous estradiol administration. Neurology 1975;25:245-250.ArticlePubMed
- 51. Somerville BW. Estrogen-withdrawal migraine. I. Duration of exposure required and attempted prophylaxis by premenstrual estrogen administration. Neurology 1975;25:239-244.ArticlePubMed
- 52. Stewart WF, Lipton RB, Chee E, Sawyer J, Silberstein SD. Menstrual cycle and headache in a population sample of migraineurs. Neurology 2000;55:1517-1523.ArticlePubMed
- 53. Verhagen IE, van der Arend BW, van Casteren DS, et al. Migraine with and without aura in relation to the menstrual cycle and other hormonal milestones: a prospective cohort study. Cephalalgia 2023;43:3331024231164322.ArticlePubMedPDF
- 54. Welch KM, Brandes JL, Berman NE. Mismatch in how oestrogen modulates molecular and neuronal function may explain menstrual migraine. Neurol Sci 2006;27 Suppl 2:S190-S192.ArticlePubMedPDF
- 55. Kudo C, Harriott AM, Moskowitz MA, Waeber C, Ayata C. Estrogen modulation of cortical spreading depression. J Headache Pain 2023;24:62.ArticlePubMedPMCPDF
- 56. Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. I. Normal pregnancy. Am J Obstet Gynecol 1972;112:1095-1100.ArticlePubMed
- 57. Hannan FM, Elajnaf T, Vandenberg LN, Kennedy SH, Thakker RV. Hormonal regulation of mammary gland development and lactation. Nat Rev Endocrinol 2023;19:46-61.ArticlePubMedPDF
- 58. Sances G, Granella F, Nappi RE, et al. Course of migraine during pregnancy and postpartum: a prospective study. Cephalalgia 2003;23:197-205.ArticlePubMedPDF
- 59. Kvisvik EV, Stovner LJ, Helde G, Bovim G, Linde M. Headache and migraine during pregnancy and puerperium: the MIGRA-study. J Headache Pain 2011;12:443-451.ArticlePubMedPMCPDF
- 60. Sacco S, Ricci S, Degan D, Carolei A. Migraine in women: the role of hormones and their impact on vascular diseases. J Headache Pain 2012;13:177-189.ArticlePubMedPMCPDF
- 61. Sacco S, Ripa P. Migraine in pregnancy. J Headache Pain 2015;16:A24.ArticlePubMedPMCPDF
- 62. Melby MK, Lock M, Kaufert P. Culture and symptom reporting at menopause. Hum Reprod Update 2005;11:495-512.ArticlePubMed
- 63. Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab 1996;81:1495-1501.ArticlePubMed
- 64. Ripa P, Ornello R, Degan D, et al. Migraine in menopausal women: a systematic review. Int J Womens Health 2015;7:773-782.ArticlePubMedPMC
- 65. Wang SJ, Fuh JL, Lu SR, Juang KD, Wang PH. Migraine prevalence during menopausal transition. Headache 2003;43:470-478.ArticlePubMed
- 66. Martin VT, Pavlovic J, Fanning KM, Buse DC, Reed ML, Lipton RB. Perimenopause and menopause are associated with high frequency headache in women with migraine: results of the American Migraine Prevalence and Prevention Study. Headache 2016;56:292-305.ArticlePubMed
- 67. Freeman EW, Sammel MD, Lin H, Gracia CR, Kapoor S. Symptoms in the menopausal transition: hormone and behavioral correlates. Obstet Gynecol 2008;111:127-136.ArticlePubMed
- 68. Makita K, Inagaki M, Kitamura S, Tatsuoka Y. Changes in migraine before and after menopause in Japanese climacteric women. Cephalalgia 2017;37:1088-1092.ArticlePubMedPDF
- 69. Oh K, Jung KY, Choi JY, Seo WK, Park KW. Headaches in middle-aged women during menopausal transition: a headache clinic-based study. Eur Neurol 2012;68:79-83.ArticlePubMedPDF
- 70. Neri I, Granella F, Nappi R, Manzoni GC, Facchinetti F, Genazzani AR. Characteristics of headache at menopause: a clinico-epidemiologic study. Maturitas 1993;17:31-37.ArticlePubMed
- 71. Kim S, Lee SB, Hong YJ, Kim Y, Han K, Park JW. The influence of endogenous and exogenous hormonal factors on migraine in spontaneous postmenopausal women: a nationwide population-based study in South Korea. Cephalalgia 2022;42:376-384.ArticlePubMedPDF
- 72. Gavrilyuk O, Braaten T, Weiderpass E, Licaj I, Lund E. Lifetime number of years of menstruation as a risk index for postmenopausal endometrial cancer in the Norwegian Women and Cancer Study. Acta Obstet Gynecol Scand 2018;97:1168-1177.ArticlePubMedPMCPDF
- 73. Chavez-MacGregor M, van Gils CH, van der Schouw YT, Monninkhof E, van Noord PA, Peeters PH. Lifetime cumulative number of menstrual cycles and serum sex hormone levels in postmenopausal women. Breast Cancer Res Treat 2008;108:101-112.ArticlePubMedPMCPDF
- 74. Aloisi AM, Bachiocco V, Costantino A, et al. Cross-sex hormone administration changes pain in transsexual women and men. Pain 2007;132 Suppl 1:S60-S67.ArticlePubMed
- 75. Greendale GA, Reboussin BA, Hogan P, et al. Symptom relief and side effects of postmenopausal hormones: results from the Postmenopausal Estrogen/Progestin Interventions Trial. Obstet Gynecol 1998;92:982-988.ArticlePubMed
- 76. Ryan RE. A controlled study of the effect of oral contraceptives on migraine. Headache 1978;17:250-252.ArticlePubMed
- 77. Merki-Feld GS, Caveng N, Speiermann G, MacGregor EA. Migraine start, course and features over the cycle of combined hormonal contraceptive users with menstrual migraine - temporal relation to bleeding and hormone withdrawal: a prospective diary-based study. J Headache Pain 2020;21:81.ArticlePubMedPMCPDF
- 78. Aegidius K, Zwart JA, Hagen K, Schei B, Stovner LJ. Oral contraceptives and increased headache prevalence: the Head-HUNT Study. Neurology 2006;66:349-353.ArticlePubMed
- 79. Cupini LM, Matteis M, Troisi E, Calabresi P, Bernardi G, Silvestrini M. Sex-hormone-related events in migrainous females. A clinical comparative study between migraine with aura and migraine without aura. Cephalalgia 1995;15:140-144.ArticlePubMedPDF
- 80. Mueller L. Predictability of exogenous hormone effect on subgroups of migraineurs. Headache 2000;40:189-193.ArticlePubMed
- 81. Granella F, Sances G, Zanferrari C, Costa A, Martignoni E, Manzoni GC. Migraine without aura and reproductive life events: a clinical epidemiological study in 1300 women. Headache 1993;33:385-389.ArticlePubMed
- 82. Hodson J, Thompson J, al-Azzawi F. Headache at menopause and in hormone replacement therapy users. Climacteric 2000;3:119-124.ArticlePubMed
- 83. Misakian AL, Langer RD, Bensenor IM, et al. Postmenopausal hormone therapy and migraine headache. J Womens Health (Larchmt) 2003;12:1027-1036.ArticlePubMed
- 84. MacGregor A. Effects of oral and transdermal estrogen replacement on migraine. Cephalalgia 1999;19:124-125.ArticlePubMedPDF
- 85. Nappi RE, Cagnacci A, Granella F, Piccinini F, Polatti F, Facchinetti F. Course of primary headaches during hormone replacement therapy. Maturitas 2001;38:157-163.ArticlePubMed
- 86. Aegidius KL, Zwart JA, Hagen K, Schei B, Stovner LJ. Hormone replacement therapy and headache prevalence in postmenopausal women. The Head-HUNT study. Eur J Neurol 2007;14:73-78.ArticlePubMed
Citations
Citations to this article as recorded by

- Migraine in pregnancy: an integrative review
María Karina Vélez-Jiménez, Pablo Schubaroff, Maria Loreto Cid-Jeffs, Ana Mercedes-Robles, Adriana Patricia Martinez-Mayorga, Ildefonso Rodriguez-Leyva, Daniel San-Juan, Javier Andrés Galnares-Olalde, Miguel Angel Morales-Morales, Kevin Espejo-Campos
Headache Medicine.2026; 16(4): 209. CrossRef - Association of diet quality, dietary acid load and antioxidant index with migraine severity, disability, and duration: a cross-sectional study
Reza Hashemi, Simin Khayatzadeh Kakhki, Hawzhin Khalid, Samira Sadat Ghalishourani, Marzieh Feyzpour, Samaneh Ansari, Hossein Gandomkar, Mohammed M. Hussien M. Raouf
BMC Neurology.2026;[Epub] CrossRef - Migraine and Multiple Sclerosis Across the Female Reproductive Lifespan: Insights for the Obstetrician-Gynecologist and Neurologists: A narrative review
Mufeed Akram Taha, Esraa Abdulkareem Mohammed, Wassan Nori
The Open Public Health Journal.2026;[Epub] CrossRef - Framing and Management of Migraines in Women: An Expert Opinion on Challenges, Current Approaches, and Future Multidisciplinary Perspectives
Piero Barbanti, Rossella E. Nappi
Healthcare.2025; 13(2): 164. CrossRef - Morning Headaches: An In-depth Review of Causes, Associated Disorders, and Management Strategies
Yooha Hong, Mi-Kyoung Kang, Min Seung Kim, Heejung Mo, Rebecca C. Cox, Hee-Jin Im
Headache and Pain Research.2025; 26(1): 66. CrossRef - Calcitonin Gene-Related Peptide Monoclonal Antibody Treatment in Nine Cases of Persistent Headache Following COVID-19-Infection
Soyoun Choi, Yooha Hong, Mi-Kyoung Kang, Tae-Jin Song, Soo-Jin Cho
Journal of Korean Medical Science.2025;[Epub] CrossRef - Validity of Migraine Diagnoses in Korean National Health Insurance Claims Data
Yoonkyung Chang, Soyoun Choi, Byung-Su Kim, Tae-Jin Song
Headache and Pain Research.2025; 26(2): 154. CrossRef - Trends in the Burden of Headache Disorders in Europe, 1990–2021: A Systematic Analysis from the Global Burden of Disease Study 2021
Terry Jung, Yoonkyung Chang, Moon-Kyung Shin, Sohee Wang, Seyedehmahla Hosseini, Joonho Kim, Min Kyung Chu, Tae-Jin Song
Journal of Clinical Medicine.2025; 14(19): 6966. CrossRef - Sex differences in migraine: bridging pathophysiology and clinical care in women
U. K. Egodage, M.S. Mohideen, S.P. Mohotti
Advances in Physiology Education.2025; 49(4): 1109. CrossRef - Migraine in Women: Inescapable Femaleness?
Soo-Kyoung Kim
Headache and Pain Research.2024; 25(1): 1. CrossRef - Three-month treatment outcome of medication-overuse headache according to classes of overused medications, use of acute medications, and preventive treatments
Sun-Young Oh, Jin-Ju Kang, Hong-Kyun Park, Soo-Jin Cho, Yooha Hong, Mi-Kyoung Kang, Heui-Soo Moon, Mi Ji Lee, Tae-Jin Song, Young Ju Suh, Min Kyung Chu
Scientific Reports.2024;[Epub] CrossRef - Subjective Cognitive Decline Patterns in Patients with Migraine, with or without Depression, versus Non-depressed Older Adults
Sun Hwa Lee, Soo-Jin Cho
Headache and Pain Research.2024; 25(2): 103. CrossRef - Understanding the Connection between the Glymphatic System and Migraine: A Systematic Review
Myoung-Jin Cha, Kyung Wook Kang, Jung-won Shin, Hosung Kim, Jiyoung Kim
Headache and Pain Research.2024; 25(2): 86. CrossRef
 , Jeong Wook Park
, Jeong Wook Park





 PubReader
PubReader ePub Link
ePub Link Cite this Article
Cite this Article
 KHS
KHS