Understanding the Connection between the Glymphatic System and Migraine: A Systematic Review
Article information
Abstract
The glymphatic system is a brain-wide perivascular pathway that functions similarly to the lymphatic system in the periphery of the body, playing a crucial role in removing waste from the brain. Although impaired glymphatic function has a well-known relationship with neurodegenerative diseases through abnormal protein accumulation, it is also associated with migraine. While still in its nascent phase, research on the glymphatic system in migraine patients is gradually increasing. This systematic literature review focuses on studies investigating the glymphatic system in migraineurs. Furthermore, it examines the methods used to evaluate the glymphatic system in these studies and their main findings.
INTRODUCTION
Migraine is one of the common primary headache and a disabling neurological disorder. Migraine is more prevalent in women compared to men, with a global prevalence ranging from 5% to 15%.1-4 The pathophysiology of migraine is still not fully understood, the new results of research are emerging. Since migraine is accompanied by pulsating headaches, in the past, the vascular theory was considered as the mechanism of development of migraine.5 Since then, research of pathophysiology on the migraine has been conducted, and recently, in a study using a preclinical migraine model, it has been found that the development of migraine is the process of the neurogenic inflammation of the trigeminovascular system. Efforts to elucidate the pathophysiology of migraines are still ongoing.6,7
Recently, a hypothesis has been proposed suggesting a potential association between the pathophysiology of migraine and impaired glymphatic system. The glymphatic system function is like the peripheral lymphatic system and the outer surface of perivascular spaces (PVS) is surrounded with astrocyte endfeet, it is termed a glymphatic system.
In 1971, Cserr proposed that both bulk flow and diffusion are necessary for maintaining solute concentration balance, removing metabolic waste, and distributing various solutes. This concept aligns with the characteristics of the glymphatic system discovered by the Nedergaard group in 2012.8 Emerging research continues to shed light on the PVS that surrounds the brain’s blood vessels.9 PVS was already described by Durand-Fardel in 1842 and Pestalozzi in 1849. Additionally, in 1851 Rudolf Virchow and in 1859 Charles Robin described the spaces surrounding vessels around brain perforating vessels, and these spaces are now also referred to as Virchow-Robin spaces, named after them.10
The lymphatic system is a complex of lymphatic vessel and lymphoid tissue that plays a crucial role in maintaining human health by regulating tissue fluid balance, transporting metabolic waste products from the interstitial fluid (ISF), and contributing to immunosurveillance and inflammation.11,12 The glymphatic system is a brain-wide perivascular pathway that acts similarly to the lymphatic system, playing a crucial role in delivering nutrients and neuroactive substances to the brain parenchyma through peri-arterial cerebrospinal fluid (CSF) influx pathway and clear cell waste of the brain through peri-venous clearance routes.13,14 This process is driven by aquaporin-4 on the endfeet of astrocytes (Figure 1). The glymphatic system serves as a pathway for removing proteins and products from the brain, and evidence suggests that when this function is impaired, the accumulation of brain proteins and products triggers inflammation, which is associated with the pathophysiology of neurological disorder in the central nervous system.15 This aspect of the glymphatic system has made it a significant topic in research on neurological disorders, including migraine.16-19 As the function of the glymphatic system is gradually revealed and research has progressed, interest in the glymphatic system in migraineurs is also increasing. Therefore, this systematic literature review is performed to assess and summarize articles regarding the glymphatic system of migraineurs.
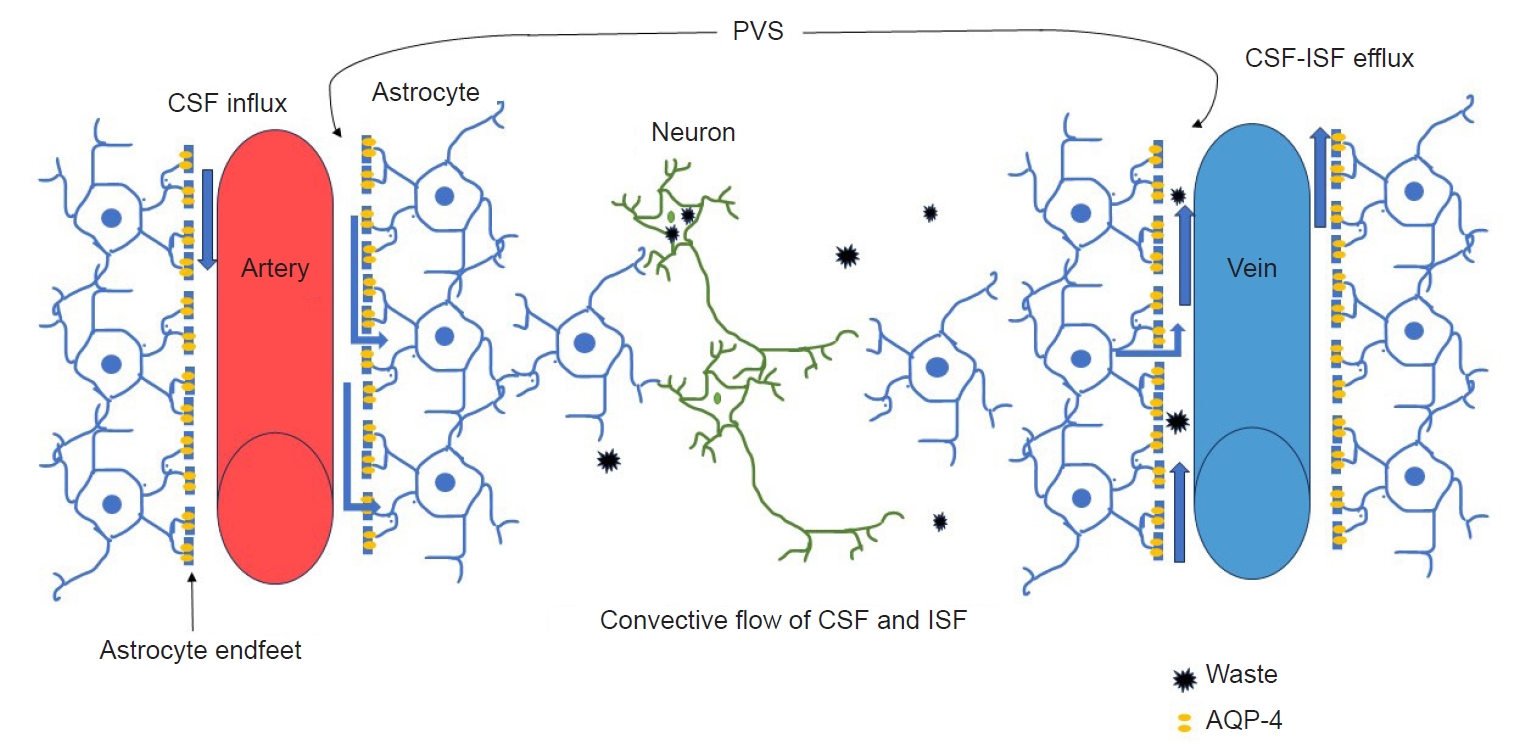
A schematic diagram of the glymphatic system structure and waste clearance function. CSF enters from the subarachnoid space through PVS around the pial arteries and penetrating arteries, and CSF enters the brain parenchyma. The flow of CSF is mediated by AQP-4, and thereafter, CSF mixes with ISF. CSF and ISF move into the perivenous space.
CSF, cerebrospinal fluid; PVS, perivascular spaces; AQP-4, aquaporin-4 channel; ISF, interstitial fluid.
METHODS
1. Data search and study selection
We performed systematic searches of the MEDLINE (from inception to March 28, 2024) and Embase (from inception to March 28, 2024) databases for publications in English using the terms “Migraine” AND (glymphatic system OR Virchow-Robin Spaces OR perivascular spaces). Articles meeting the predetermined selection criteria were retrieved for full-text screening to determine eligibility (Table 1). All searches were limited to human studies. Review articles, case reports, abstracts, and editorials were excluded, and duplicate data were removed. When more than one study with a similar topic was published from the same institution, only the report with the highest number of patients relevant to this study was included. Two authors independently searched the databases, screened potential studies, and reviewed the data. Discrepancies were resolved by consensus.
2. Data extraction and analysis
Data were extracted using a pre-defined form, including the following: first author, year of publication, country, study design, total number of cases, study characteristics of participants, and main outcome.
RESULTS
1. Study and participant characteristics
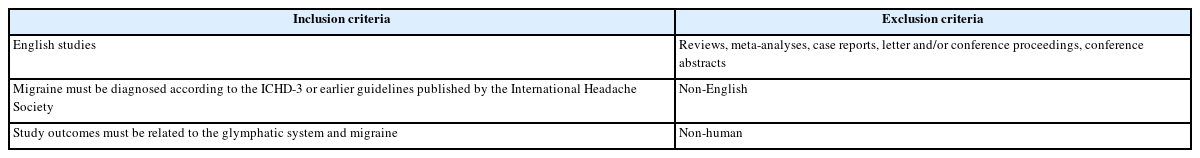
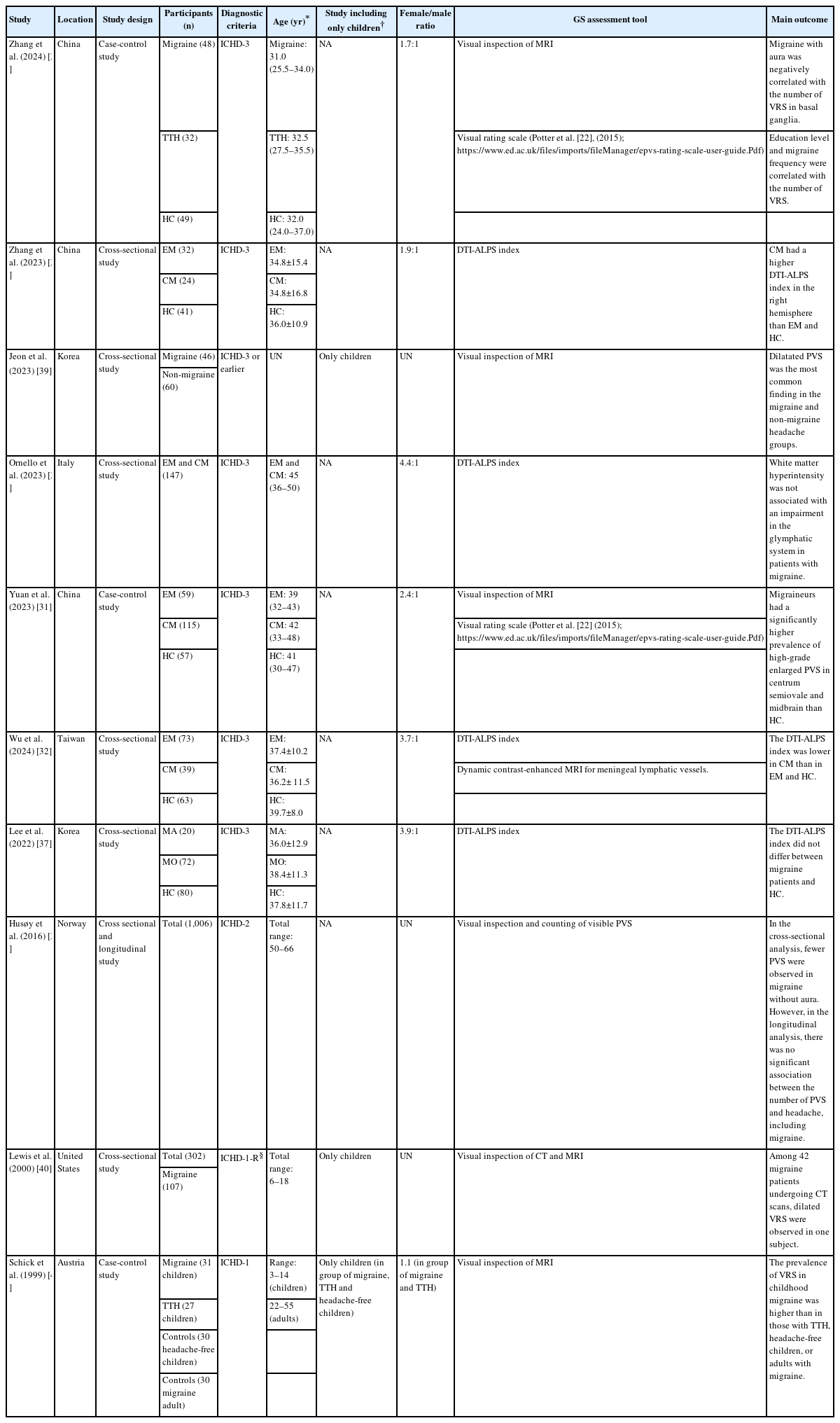
Figure 2 shows the studies included in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart. Ten studies were included in the final analysis. Many studies were conducted on adults with migraine. However, three studies primarily focused on children with migraine. Additionally, due to the epidemiological characteristic of migraine being more common in females, there was a higher proportion of females among the study participants (Table 2).
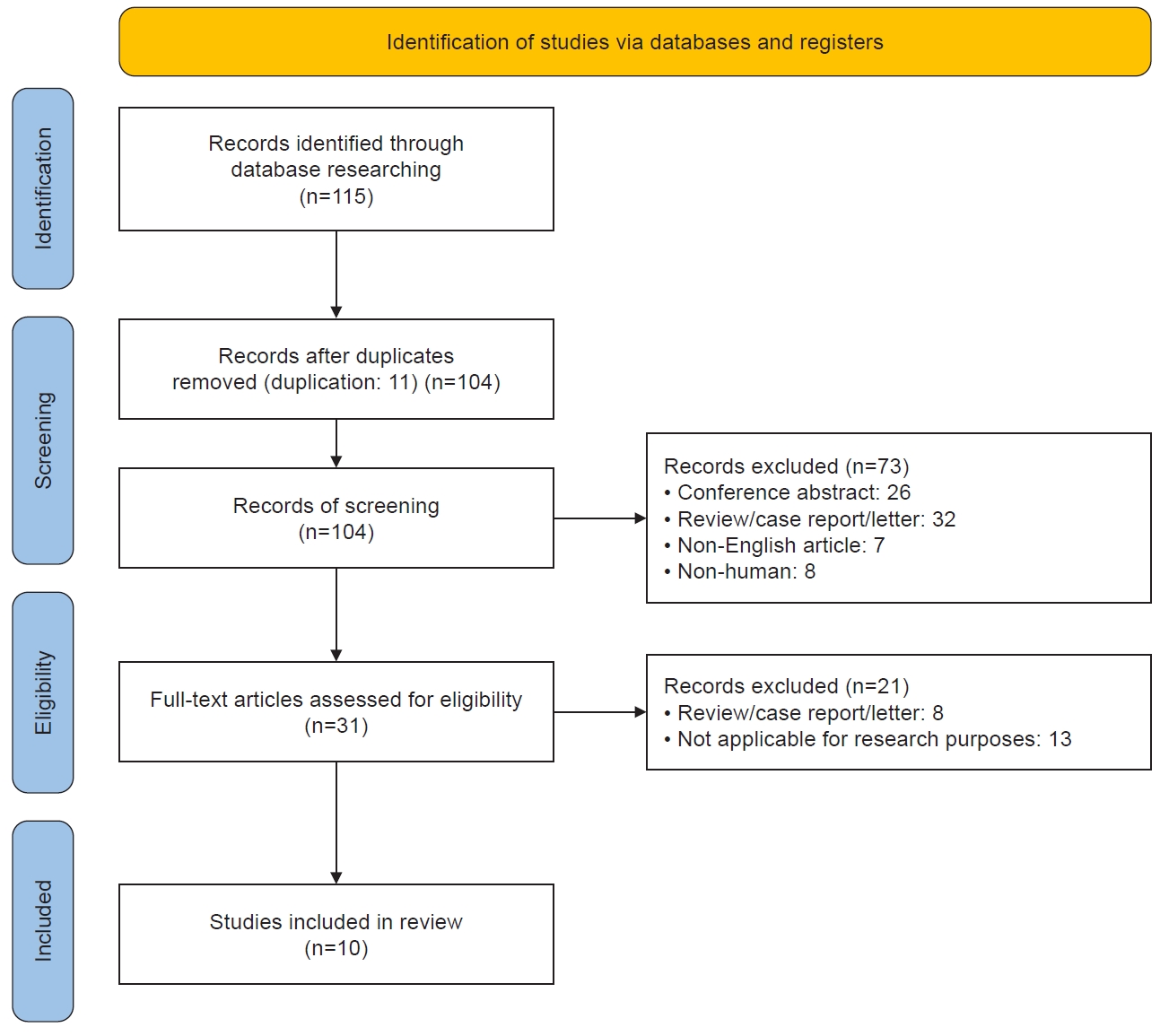
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for included studies. A total of 115 studies were identified through the search, and 10 studies met the inclusion criteria.
2. Assessment method of glymphatic system
The methods for evaluating the glymphatic system have varied depending on the researchers and the characteristics of the participants of the study. Particularly, there have been significant differences depending on the time of the study, as the evaluation methods for the glymphatic system rely on brain imaging technology, and these assessment methods inevitably change with the advancement of brain imaging technology.20 Some studies have been conducted by experienced neuroimaging experts evaluating the number or shape of visible PVS. Recent studies have applied the methods proposed by Potter et al.21 and Taoka et al.22 Both methods evaluate alterations in glymphatic system function, but they are somewhat different. The method proposed by Potter et al.21 quantifies the number of enlarged perivascular spaces (EPVS), which become prominent when the glymphatic system is functionally impaired. In contrast, the approach by Taoka et al.22 evaluates glymphatic function by measuring the diffusion rate of water molecules along the PVS.
Potter and colleagues suggest the visual rating scale of visible EPVS on magnetic resonance imaging (MRI) and user guide (https://www.ed.ac.uk/sites/default/files/imports/fileManager/epvs-rating-scale-user-guide.pdf). The non-invasive method of assessing the EPVS observed on MRI reveals abnormalities in glymphatic system drainage.23 EPVS is defined as small and sharply delineated structures of isointense to CSF, measuring less than 3 mm, that follow perforating vessels on MRI in this method. EPVS is easily observed on T2-weighted images. The method proposed by Potter et al.21 evaluates EPVS in three different regions (centrum semiovale [CSO], basal ganglia [BG], and midbrain [MB]) and proceeds as follows: Both sides of every MRI slice in each region are observed, and the number of EPVS is recorded for the hemisphere with more EPVS. Subsequently, the slice showing the highest number of EPVS is selected, and the level of EPVS is categorized based on the observed number of EPVS, using classification criteria in each region. Previous studies reported that EPVS burden is associated with cerebral small-vessel disease, Alzheimer’s disease, and Parkinson’s disease.21,24,25
Taoka et al.22 proposed the diffusion tensor image analysis along the perivascular space (DTI-ALPS) method, which offers the advantage of non-invasively assessing the activity of the glymphatic system. In this method, the function of the glymphatic system is expressed as the ALPS index. DTI-ALPS measures the movement of water molecules in the direction of PVS at the level of the lateral ventricle body. A larger DTI-ALPS index indicates a larger diffusivity of water along the PVS, while a smaller DTI-ALPS index indicates a smaller diffusivity of water along the PVS. According to previous studies, the DTI-ALPS index is significantly decreased in aging, Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis, indicating a decrease in glymphatic activity and impairment of glymphatic clearance function in these conditions or diseases.26-29
3. Glymphatic system and perivascular spaces in adult with migraine
Some studies conducted on adults with migraine have evaluated the glymphatic system and PVS among chronic migraine (CM), episodic migraine (EM), and healthy controls (HC).30-32 In the study by Zhang et al.30 using the DTI-ALPS index, the CM showed a significantly higher DTI-ALPS index in the right hemisphere compared to the EM and HC. Wu et al.32 also investigated the glymphatic system by using the DTI-ALPS index. In this study, the DTI-ALPS index of CM is significantly lower than those of EM and HC. When comparing the ALPS index of CM with those of EM and HC, discrepancies were observed between the results of the two studies. However, both studies speculated that the significant changes in the DTI-ALPS index observed in CM may be attributed to alterations in the activity of the glymphatic system associated with calcitonin gene-related peptide (CGRP). Yuan et al.31 investigated EPVS utilizing a 3-T MRI employing a validated visual rating scale. In this study, it was observed that participants with EM and CM exhibited a higher grade of EPVS in the CSO and MB regions, indicative of a greater number of EPVS, compared to HC. The observed increase in the number of EPVS in migraine is presumed to be associated with dysfunction of the glymphatic system, potentially attributed to neurogenic inflammation of migraine pathophysiology.
EPVS and white matter hyperintensity (WMH) are known as radiological features of cerebral small vessel disease.33 While further research is still needed, some reports are suggesting a significant increase in the prevalence of WMH in migraine.34 Two studies have focused on WMH and PVS as primary topics in migraine patients.35,36 In one study, the presence of WMH, considered a key feature of cerebral small vessel disease, was investigated for its association with dysfunction in the glymphatic system in patients with migraine.35 This research compared the function of the glymphatic system, using the DTI-ALPS index, between migraine patients with and without WMH. The results indicated no significant difference in the DTI-ALPS index between migraine patients with and without WMH, and did not demonstrate a significant association between WMH and dysfunction in the glymphatic system in migraine patients. However, this study proposed the necessity for further investigation by applying alternative evaluation methods to explore the function of the glymphatic system in migraine patients with WMH. Additionally, this study also suggested that further studies on the characteristics of WMH in migraine patients are needed. In another study comparing migraine, tension-type headache, and HC groups, the occurrence of WMH was significantly higher in the migraine group compared to the tension-type headache and HC group.36 The Scheltens score indicating the extent of WMH and the total volume of WMH also showed significant differences when comparing the migraine group with the tension-type headache and HC group, respectively. Additionally, the study reported number of visible PVS was associated with aura, headache frequency, and educational level.
One pilot study evaluated glymphatic dysfunction using the DTI-ALPS index between migraine and HC.37 This study specifically compared the function of the glymphatic system between migraine with aura and migraine without aura. The results indicated no significant difference in glymphatic system function between the HC and migraine, as well as between migraine with aura and migraine without aura.
Research findings on visible PVS in migraine, including longitudinal analysis, are very limited. The HUNT-MRI study performed both cross-sectional and longitudinal analysis.38 In the cross-sectional analysis, visible PVS was fewer in patients with migraine compared to those without headache. However, in longitudinal analysis, there was no significant difference in visible PVS between headache patients including migraine, and those without headache. Thus, the study reported no association between headache and visible PVS.
4. Glymphatic system and perivascular spaces in child with migraine
The research findings regarding glymphatic function in pediatric migraine are scarce. In a study examining brain MRI of children with primary headaches, dilated PVS larger than 2 mm were the most frequently observed MRI findings, with the BG being the most common location.39 Moreover, this study reported no significant differences in PVS size, location, and shape between the migraine and non-migraine groups. In a study analyzing brain computed tomography (CT) scan and MRI results in children with migraine, findings from CT scans of 42 out of 107 migraine patients were reported.40 This study showed dilated Virchow-Robin spaces were observed in one patient among 42 children with migraine. In a comparative study among pediatric migraine, pediatric tension-type headache, pediatric non-headache, and adult non-headache groups, pediatric migraine group showed a significantly higher prevalence of visible PVS compared to the other three groups.41
5. Evidence linking glymphatic dysfunction to migraine
The connection between migraine and the glymphatic system may be explored from the following four perspectives42,43: 1) neuroinflammation, 2) CGRP, 3) cortical spreading depression (CSD), and 4) sleep.
In the context of neuroinflammation, the glymphatic system is involved in clearing reactive oxygen species (ROS) and ROS-related inflammation.44 If the glymphatic system is impaired, ROS may not be adequately cleared, leading to an increase in proinflammatory cytokines such as tumor necrosis factor-α, interleukin-1 β, and hypoxia-inducible factor-1α released by microglial cells.45,46 The accumulation of ROS and pro-inflammatory cytokines exacerbates neuronal degeneration and astrocyte damage, making a vicious cycle that worsens glymphatic system abnormality.47 Moreover, pro-inflammatory cytokines can activate nociceptors, increasing pain sensitivity.48 These neuroinflammation processes suggest a potential link between migraine and glymphatic dysfunction.
CGRP, a neuropeptide and vasodilator, is known to play a significant role in the pathogenesis of migraine. Released from trigeminal afferent fibers surrounding pial arteries and veins, the CGRP can move PVS around cortical arteries through the CSF, entering the interstitial spaces and PVS surrounding cortical veins. Following this pathway, CGRP may collect in lymphatic vessels in the superior sagittal sinus.49 This process leads to the hypothesis that dysfunction of the glymphatic system may accumulate the CGRP and thus worsen migraine.
Impaired glymphatic system flow induced by CSD was observed in an animal model study. This investigation revealed that CSD rapidly and nearly completely closed the PVS surrounding cortical arteries and veins, impairing the clearance of ISF from the cortex to the PVS. These findings also suggest an association between migraine and the glymphatic system.19
Finally, sleep may play a crucial role in linking migraine and the glymphatic system. Sleep disorders including insomnia are risk factors for migraine, whereas sleep can be a means of terminating migraine. A hypothesis has been proposed suggesting that sleep deprivation reduces glymphatic system clearance, leading to the accumulation of headache-related substances in the ISF. These conditions can be considered to lower the threshold for headaches, potentially contributing to the chronicity of headaches, including migraines. Sleep, during which the glymphatic system functions actively, can terminate migraine, while sleep disorders that may limit the function of the glymphatic system can trigger migraine. This suggests that the function of the glymphatic system may be associated with migraine through the mediation of sleep.43
6. Clinical implications of understanding the glymphatic system and migraine connection
Animal and human studies have suggested the association between migraine and glymphatic system dysfunction in recent years. However, this field is still in its early stages. If the relationship between migraine and glymphatic system dysfunction becomes clearer, new drugs targeting glymphatic system regulation may emerge as preventive or acute treatment options for migraine. Interestingly, adrenergic inhibitors like propranolol which are used as preventive medications for migraine, have been found in animal study to increase the interstitial volume of the glymphatic system, promoting the flow and clearance of glymphatic ISF.50,51 While further research is needed, this suggests the possibility of prevention of migraine by regulating glymphatic system function.
CONCLUSION
This systematic literature review provided insights into recent research findings on the glymphatic system related to migraine. After this systematic review, the main findings can be summarized into the following three aspects. Firstly, recent studies have utilized the DTI-ALPS index or structured visual rating scales to evaluate glymphatic system function. Secondly, changes in glymphatic system function in migraine are not consistently reported due to limited research findings, but impairment or alterations in glymphatic system function are suspected in CM. Thirdly, studies evaluating the glymphatic system in migraine patients with longitudinal designs or focusing on pediatric populations are limited. Since the elucidation of the glymphatic system function in the brain, various neurological disorders have become research topics. However, research findings on the glymphatic system and migraine remain insufficient, emphasizing the continued need for studies focusing on migraine animal models and migraine patients.
Notes
AVAILABILITY OF DATA AND MATERIAL
Not applicable.
AUTHOR CONTRIBUTIONS
Conceptualization: JK; Data curation: MJC, JK; Formal analysis: MJC, JK; Funding acquisition: JK; Investigation: MJC, JK; Methodology: MJC, JK; Writing–original draft: MJC, JK; Writing–review and editing: MJC, KWK, JWS, HK, JK.
CONFLICT OF INTEREST
Jiyoung Kim is the editor of Headache and Pain Research and was not involved in the review process of this article. All authors have no other conflicts of interest to declare.
FUNDING STATEMENT
This study was supported by the 2023 overseas training grant from Pusan National University Hospital.
ACKNOWLEDGMENTS
Not applicable.