Subjective Cognitive Decline Patterns in Patients with Migraine, with or without Depression, versus Non-depressed Older Adults
Article information
Abstract
Purpose
Cognitive decline is a common complaint in young patients with migraine, especially those with depression. Independent of psychiatric factors such as depression, subjective cognitive decline (SCD) is associated with an elevated risk of progression to dementia. This study aimed to investigate patterns of subjective cognitive complaints between migraineurs with or without depression and non-depressed older adults.
Methods
This retrospective study included 331 outpatients with SCD (293 from a headache clinic and 38 from a memory clinic). SCD was diagnosed as “yes” based on two questions about SCD. The Mini Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) were used to assess cognitive function. The SCD Questionnaire (SCD-Q) with three subdomains was analyzed to compare SCD between groups.
Results
Among patients with SCD, significant differences in duration of education were found among the groups—specifically, migraineurs with depression (12.39 years) had longer education than non-depressed older adults (10.50 years) and shorter education than migraineurs without depression (14.28 years). The total MMSE and MoCA scores did not differ between migraineurs with and without depression. Regarding SCD-Q scores, migraineurs with depression showed higher scores overall and in all cognitive domains than migraineurs without depression, with no significant difference compared to non-depressed older adults.
Conclusion
Although the depressed migraineurs with SCD were younger and more educated than the non-depressed older adults with SCD, both groups reported similarly high levels of SCD. Higher levels of surveillance for cognitive decline are warranted for migraineurs with depression who have SCD.
INTRODUCTION
Given the escalating dementia crisis within the population, it is crucial to study the conditions associated with cognitive decline and evaluate interventions to mitigate this growing burden.1 With the success of β-amyloid treatment, the importance of evaluating early detection and intervention at the pre-clinical stage is also increasing, particularly in cases of APOE ɛ4, a known genetic risk factor for Alzheimer’s disease (AD).2 Subjective cognitive decline (SCD) is a clinical state characterized by reports of self-perceived persistent worsening of cognitive function, despite scores remaining within normal ranges on standardized tests.3 Emerging evidence from longitudinal studies indicates that older adults with SCD are more likely than their healthy peers to exhibit AD biomarkers such as neurodegeneration4,5 and amyloid burden.6,7 A recent systematic review on SCD and APOE4 found a relationship between APOE ɛ4, SCD, and AD, which implies SCD to be considered as preclinical AD.8 It suggests that, for some older adults, SCD may represent a preclinical phase of AD. A recent meta-analysis study reported that higher depressive symptoms in SCD does not elevate risk of progression to dementia.9
Migraine is another highly prevalent neurological disorder widely recognized for its pathophysiology involving various cortical and subcortical brain structures and associated with various comorbidities.10-12 Although cognitive symptoms are not the primary concern for patients with migraine, since both conditions originate from brain, complaints of cognitive impairment are often observed in clinical settings and are considered to be manageable symptoms.13 Despite contradictory evidences of the association between migraine and cognitive impairment,14,15 and a recent narrative review on cognitive impairment in headache disorder found that migraine is associated with cognitive impairment years before a migraine diagnosis and is unlikely that migraine is associated with dementia.16 Therefore, although the final outcomes may completely differ, whether for dementia or migraine, concerns about cognitive function can be the earliest sign to emerge before an actual neurological disorder is diagnosed.
We reported that subjective complaints of cognitive impairment without actual impairment are also common in young patients with migraine, and many of them also reported mood and sleep complaints.13 Within the framework of SCD, the underlying factors that elicit subjective cognitive symptoms might differ between older adults without migraine and younger adults with headache. For the older adults, SCD is the primary concern, whereas for younger adults with headache, headache itself is the primary concern, with SCD being a supplementary symptom. However, given that the major clinical presentations are different, the symptoms constituting SCD may vary between the two groups. Identifying specific patterns of cognitive decline within each group is believed to be clinically meaningful for detailed planning non-pharmacological treatment. This study was aimed to investigate the patterns of subjective cognitive complaints between “non-depressed older adults” and “patients with migraine with or without depression.”
MATERIALS AND METHODS
1. Participants
The present study was based on a retrospective review of medical records collected from 2016 to 2017 from the first visit to either Headache Clinic or the Memory Clinic at the Department of Neurology, Hallym University Dongtan Sacred Heart Hospital.
In the case of memory clinic visitors, the inclusion criteria were as follows: 1) patients aged 60 years or older who voluntarily presented to the hospital with major complaints of memory decline, 2) said “yes” to both the following questions; a) do you perceive memory or cognitive difficulties?, b) in the last 2 years, has your cognition or memory declined?, 3) A comprehensive cognitive test revealed normal cognitive function, 4) patients who did not report depression symptoms, 5) patients who agreed to take part provided informed consent, 6) patients completed the subjective cognitive decline Questionnaire (SCD-Q) for pattern analysis.17
In the case of headache clinic visitors, the inclusion criteria were as follows: 1) patients who presented voluntarily to the hospital with major complaints of headache, 2) diagnosis of episodic migraine with or without aura, or diagnosis of chronic migraine, 3) said “yes” to both the following questions; a) do you perceive memory or cognitive difficulties?, b) in the last 2 years, has your cognition or memory declined?, 4) patients who agreed to take part provided informed consent, 5) patients completed the SCD-Q17 and Patient Health Questionnaire-9 (PHQ-9).18
Participants who did not meet the inclusion criteria were excluded. The diagnosis of headache disorders was based on the beta version of the International Classification of Headache Disorder, 3rd edition.19
The study protocol was reviewed and approved by the Institutional Review Board of the Hallym University Dongtan Sacred Heart Hospital (No. 2017-10-004) and allowed of additional written informed consent to be waived due to retrospective data collection and fully anonymity.
2. Assessments and procedures
Demographic data such as age, sex, years of education were collected. For the memory clinic population, the Seoul Neuropsychological Screening Battery 2nd edition (SNSB-II) was used to examine cognitive function.20 This battery consists of a total of 29 subtests that assesses five cognitive domains: attention, language, visuospatial function, memory, and frontal/executive function. Patients who performed 1 standard deviation below the normative values for their age and education level were operationally defined as having impairment on the subtest. For the memory clinic participants, the Short form of Geriatric Depression Scale (SGDS),21 included in the SNSB-II, was utilized to assess depression. A cutoff score of 8 was used to determine the presence of absence of depression. Participants scoring below 8 were included in the study. The final included participants were designated as the non-depressed elderly adult SCD (EA-SCD) (Figure 1A).
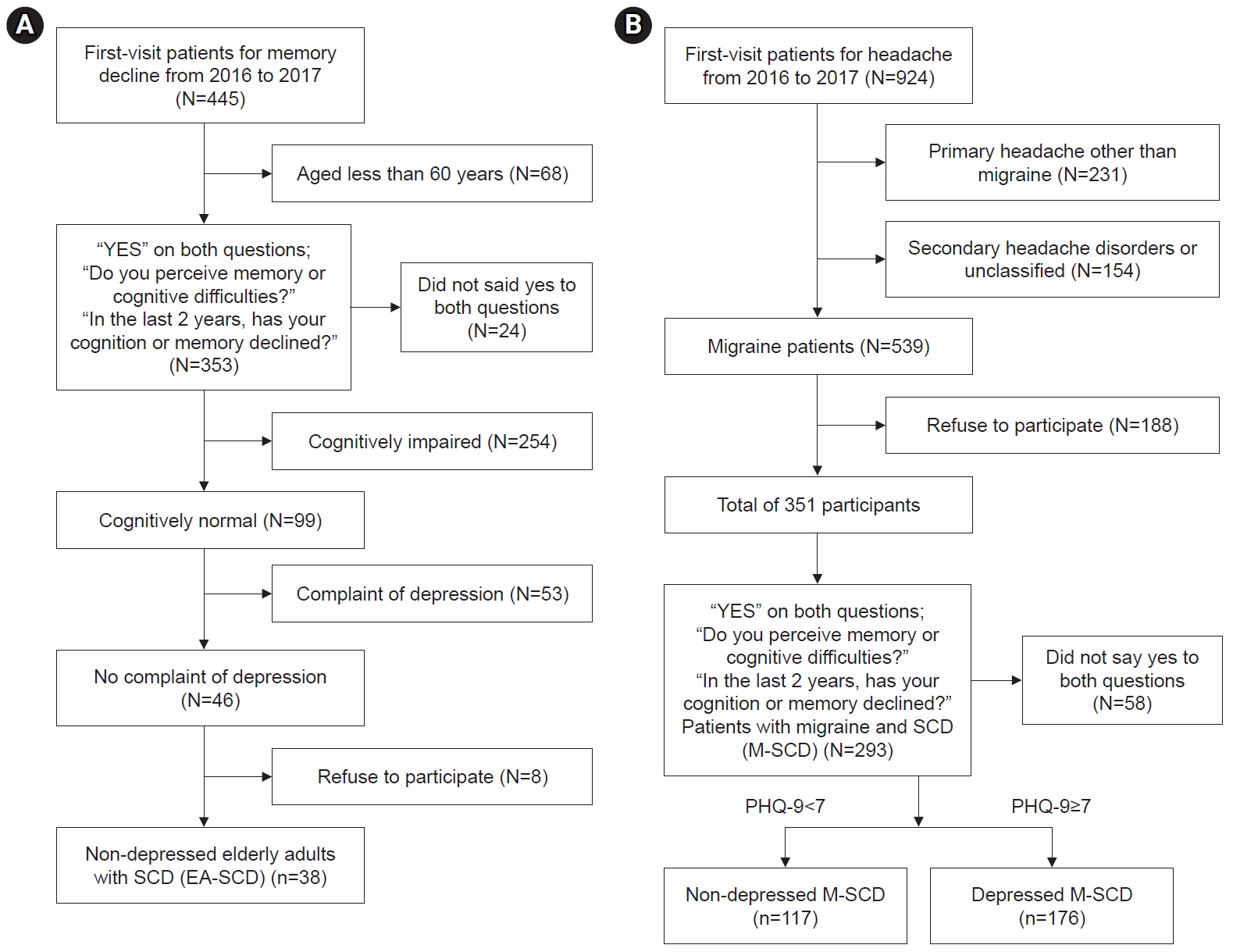
Flow diagram of sample selection: (A) Non-depressed elderly adults with SCD (EA-SCD). (B) Non-depressed and depressed patients in the migraine with SCD (M-SCD) group.
SCD, subjective cognitive decline; PHQ-9, Patient Health Questionnaire-9.
The characteristics of the headache were not collected for this study as they were not the primary focus of the analysis. Participants who agreed to take part in this study also completed two screening tests, the Korean-Mini Mental State Examination (K-MMSE)22 and the Korean-Montreal Cognitive Assessment (MoCA),23 to evaluate cognitive impairment. Patients who performed 1 standard deviation below the normative values for their age and education level were operationally defined as having cognitive impairment. For the headache clinic participants, the PHQ-9 was utilized to assess depression. A cutoff score of 7 was considered sensitive and specific to detect MDD in a patient population with migraine. The final included participants were designated as either the non-depressed or depressed patients with migraine SCD (M-SCD) group (Figure 1B).
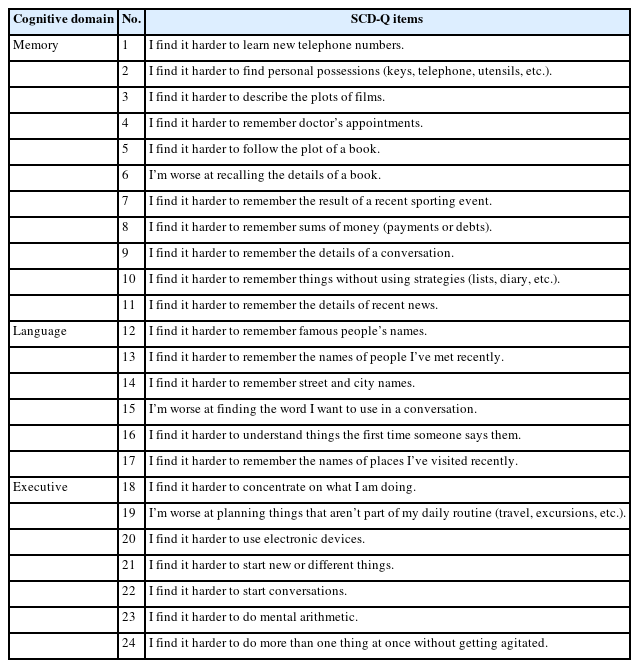
The SCD-Q17 was utilized to investigate the patterns of subjective cognitive complaints among the groups. The SCD-Q consists of 24 items that assess three cognitive domains: memory (11 items), language (6 items), and executive functions (7 items), as outlined in Table 1. The response to each question was restricted to “yes” or “no” based on individual’s perceived decline in each item. For the pattern analysis, the mean of the total SCD-Q score and the scores for each cognitive domain were compared.
3. Data analysis
Microsoft Excel 2010 and PASW statistics for Windows (version 18.0; IBM Corp.) were used for data analysis. A one-way ANOVA with Bonferroni correction was conducted for continuous variables, and Pearson’s chi-squared test for categorical data were performed for categorical data.
RESULTS
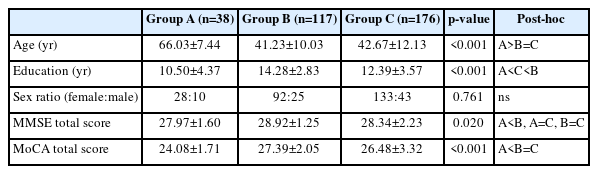
Demographic and cognitive screening test results are shown in Table 2. The difference in sex ratio among the groups was not significant, with all groups having a higher proportion of females. Mean age of the EA-SCD group was significantly higher than that of both of the M-SCD groups. However, there was no significant age difference between the M-SCD groups with and without depression. Significant differences in duration of education were found among the groups. Non-depressed M-SCD had the highest education duration, followed by depressed M-SCD, with non-depressed EA-SCD having the lowest education duration. The total scores of the K-MMSE for non-depressed EA-SCD was significantly lower than those of the non-depressed M-SCD. The total scores of MoCA for non-depressed EA-SCD was significantly lower than those of both M-SCD groups (Table 2).
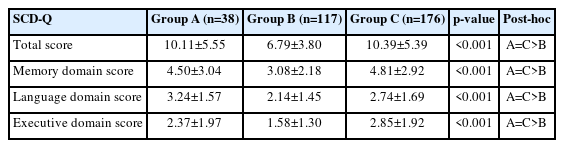
There was no significant group difference between non-depressed EA-SCD and depressed M-SCD in the total and all three cognitive domain scores of the SCD-Q. However, these two groups showed significantly higher scores in the total and all three cognitive domain scores compared to non-depressed M-SCD (Table 3).
DISCUSSION
We explored the pattern differences of cognitive profile among the individuals with SCD in memory clinic and headache clinic using the SCD-Q.17 The main finding was that the patterns of cognitive complaints were similar between the non-depressed older adults who visited our memory clinic and the depressed younger migraine patients who visited our headache clinic. The total score and three cognitive domain scores of both groups were significantly higher than those of non-depressed younger migraine patients. Upon closure examination, the total scores of SCD-Q of non-depressed older adults and depressed younger migraine patients were 10.11±5.55 and 10.39±5.39, respectively, both exceeding the suggested cutoff value of 8. However, in the case of the non-depressed younger migraine patients who visited our headache clinic, the mean total score was 6.79±3.80, which was lower than the suggested cutoff value. Therefore, using only two simple questions about one’s subjective perception of cognitive decline appears to be insufficient for screening patients with SCD.
Despite the depressed migraine patients being younger, more educated, and scoring higher on the MoCA than the non-depressed older adults, both groups reported similarly high levels of SCD. There is a possibility of ceiling effect since we did not use a comprehensive neuropsychological test but rather a short screening test for objective cognitive assessment. However, it is meaningful that all patients demonstrated performance within the normal range for their expected age and education level according to normative data. A recent meta-analysis reported that although higher depressive symptoms in SCD do not elevate the risk of dementia but higher anxiety symptoms or SCD-related worry do elevate the risk.9 Together with our findings, this indicates that individuals traditionally labeled as the ‘worried well’ are at an increased risk of progression to dementia if their primary concern is cognitive deficit, or to increase comorbidity with depression in patients with migraine. This group may benefit from interventions targeted at reducing worry. We conducted a preliminary study with a total of five female patients with migraines who completed five to seven sessions of mindfulness-based cognitive therapy classes over 2 months.24 We found it to be helpful in reducing frequency of headache and medication uses, as well as alleviating emotional burdens of depression and anxiety in patients with migraine.
Upon closure examination between the non-depressed older adults who visited our memory clinic and the depressed younger migraine patients who visited our headache clinic, it was found that although the group difference was not statistically significant, the total score and the memory and executive function domain scores were slightly higher for the depressed younger migraine patients compared to the non-depressed older adults. The only cognitive domain score that was slightly higher for the non-depressed older adults appears to be on language function. In our results, the presence of depression may function as a moderating factor, contributing to increased levels of complaints about memory, language, and executive dysfunction in patients with migraines.
A recent meta-analysis of 17 articles published up to January 2020 showed the possible association between migraine and cognitive dysfunction interictally.25 Patients with migraine had worse performance in complex attention (12 studies), memory (5 studies), and visuospatial function (6 studies). A recent qualitative study revealed that patients with migraine reported problems in language, sustained attention and executive function.26 The data was collected through 90-minute, in-depth, semi-structured qualitative interviews. However, we found similar results using a short questionnaire more appropriate for clinical use, where depressed young migraine patients reported significant declines in their memory, language, and executive function. Contrary to the expectation that young individuals with depression would predominantly report cognitive decline, it appears that young migraine patients can experience and report cognitive decline even in the absence of depressive symptoms. Therefore, incorporating cognitive function assessments alongside emotional evaluations in these patients could be particularly beneficial in developing more specific and detailed treatment plans, especially for non-pharmacological interventions. Providing patients who complain of SCD, but do not exhibit actual cognitive impairment, with more accurate and objective information about their cognitive function is expected to moderate their symptoms. This represents a key clinical implication of this study.
The main limitation is the possibility of sampling bias, as the study is based on a retrospective review of headache registry records drawn from a single hospital. Different depression scales were used depending on the recruiting site: the SGDS was employed in the memory clinic, while the PHQ-9 was used in the headache clinic. Additionally, the SNSB-II was administered exclusively in the memory clinic to assess whether the cognitive function of the subjects was within normal limits. Comprehensive neuropsychological tests like the SNSB-II were not administered to patients in the headache clinic. The choice of depression scale was made to optimize the screening process based on the patient’s chief complaint and age. These scales were used solely as criteria for determining study inclusion, according to the cutoff values specified by each scale. Although comparing depression levels between groups was not the primary objective of this study, using the same scale across all groups could potentially provide richer and more comparable data regarding depression levels. Prospective data drawn from multiple centers would be necessary in future. It is also possible that the mood and/or cognitive problems reported by migraine patients were due to side effects of treatment, a result of ineffective treatment, or unrelated to treatment. Therefore, exploring information on treatment period, types, mediation, and its satisfaction could provide more valuable insights into this population.
Notes
AVAILABILITY OF DATA AND MATERIAL
Anonymized data supporting the findings presented in the current study will be shared upon reasonable request from a qualified investigator.
AUTHOR CONTRIBUTIONS
Conceptualization: SHL, SJC; Data curation: SHL; Investigation: SHL; Writing–original draft: SHL; Writing–review & editing: SHL, SJC.
All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
Soo-Jin Cho is the Editor-in-Chief of the Headache and Pain Research since 2023 and was not involved in the review process of this article. All authors have no other conflicts of interest to declare.
FUNDING STATEMENT
Not applicable.
ACKNOWLEDGMENTS
Not applicable.