Bilateral Greater Occipital Nerve Block for Preventing the OnabotulinumtoxinA Wear-off Phenomenon in the Treatment of Chronic Migraine: A Case Series of 12 Patients
Article information
Abstract
Purpose
OnabotulinumtoxinA is widely used to treat chronic migraines; however, the wear-off phenomenon before the next scheduled dose has emerged as a challenge. This study suggests a new strategy for preventing the wear-off phenomenon using bilateral greater occipital nerve block.
Methods
We conducted a retrospective review of patients diagnosed with chronic migraine who were treated with onabotulinumtoxinA and bilateral greater occipital nerve block at St. Vincent Hospital from January 2023 to December 2023. Twelve chronic migraine patients with a history of the wear-off phenomenon received a greater occipital nerve block 8 weeks after the initial onabotulinumtoxinA injection for two sessions. Responses to treatment were evaluated with regular follow-ups and daily headache diaries.
Results
All patients who had previously experienced the wear-off phenomenon with conventional onabotulinumtoxinA treatment did not experience the wear-off phenomenon during two sessions with an additional greater occipital nerve block administered 8 weeks after each onabolulinumtoxinA injection.
Conclusion
Bilateral greater occipital nerve block administered 8 weeks after the initial onabotulinumtoxinA injection effectively prevents the wear-off phenomenon, enabling sustained therapeutic benefits in chronic migraine patients. Further research is needed to confirm these findings in larger cohorts.
INTRODUCTION
OnabotulinumtoxinA is widely used as a treatment for chronic migraine (CM).1 The well-known studies, Phase III Research Evaluating Migraine Prophylaxis Therapy (PREEMPT) trials already demonstrated that onabotulinumtoxinA significantly reduced the frequency of headache days and improved quality of life in patients with CM.2 Even though, newly developed medication such as calcitonin gene-related peptide (CGRP) monoclonal antibody drugs are increasing in their uses, onabotulinumtoxinA is still important treatment option for CM.3
However, one challenge that has arisen with the use of onabotulinumtoxinA is the occurrence of drug wear-off phenomenon, which is characterized by an initial period of effectiveness followed by a reduction in therapeutic benefits before the next scheduled dose. Like the phenomenon observed with the intake of other conventional medications, wear-off was not exempt in the case of onabotulinumtoxinA administration.
The definition of the onset of the wear-off phenomenon varies from study to study. There is no academic agreement regarding the cut-off point of which week we should consider that the patient has wear-off phenomenon. Although some studies delay it to week 10,4 most authors consider week 8 as the cut-off point, defining wear-off phenomenon as the worsening of headache that occurred during the 4 weeks prior to the scheduled reinjection of onabotulinumtoxinA.5-7 A retrospective chart review of 143 patients revealed that wear-off phenomenon most commonly occurred 4 weeks before the next injection.8
Additional studies have been conducted to explore the frequency and patterns associated with wear-off phenomena related to onabotulinumtoxinA administration. Recent studies have shown that this wear-off phenomenon affects up to two-thirds of patients and occurred in especially first and second sessions of onabotulinumtoxinA administration.8 However, the exact cause of wear-off remains uncertain, as no variations in patient traits have been identified as reliable predictable factors of this phenomenon.5
Therefore, we need to consider some options to overcome this wear-off phenomenon. One option may be increasing the dosage administered at the onset of treatment to minimize the necessity for additional bridging therapy.8 Adherence to strict 12-week interval, which means not exceeding 12 weeks like roughly 3-month interval, may be also advisable. In addition, adopting a more frequent treatment schedule could potentially enhance effectiveness.6
In addition to these approaches, we came up with the idea of preventing wear-off by administering preventive nerve blocks at appropriate times. Nerve blocks, particularly greater occipital nerve (GON) blocks, have become an important therapeutic option for managing various types of headaches, including CM. These procedures involve the targeted injection of anesthetics or steroids to interrupt pain signals, providing significant relief and reducing headache frequency and severity.9
There are several studies about using GON block for migraine treatment. GON blocks for CM patients demonstrated significant efficacy in reducing migraine frequency and severity.10 Furthermore, significant decrease in number of attacks in acute migraine have also been demonstrated.11
This study has been conducted to investigate whether the effects of nerve blocks can be utilized to relieve the wear-off phenomenon observed in onabotulinumtoxinA treatment for CM.
MATERIALS AND METHODS
The objective of this study was to evaluate the effect of bilateral GON block for preventing onabotulinumtoxinA wear-off phenomenon in CM patients. This study was conducted under an approval by The Catholic University of Korea, St. Vincent’s Hospital Institutional Review Board (number: VC24RIS10166).
1. Study population and eligibility
We conducted a retrospective review of patients diagnosed with CM treated with onabotulinumtoxinA and bilateral GON block at St. Vincent Hospital from January 2023 to December 2023. Patients included in the analysis had a diagnosis of CM, required onabotulinumtoxinA treatment for recurrence, and had a history of the wear-off phenomenon during previous onabotulinumtoxinA treatment. The inclusion criterion of a history of wear-off during previous injections suggests that these patients may be particularly susceptible to experiencing wear-off with the current injection as well. Another inclusion criterion for this study was that patients should not have received a recent onabotulinumtoxinA injection within the last 6 months to exclude any remaining effects of the previous injection.
2. Method
Treatment with onabotulinumtoxinA was conducted by one experienced professional at strict 12-week intervals, following the PREEMPT injection paradigm, which administers 155 units using a standardized method at each injection session. Week 1 after onabotulinumtoxinA injection was defined to include days 1–7 post-injection, week 2 as days 8–14, and so on. The nerve block was performed 8 weeks after the first and second sessions of onabotulinumtoxinA injection. Using an ultrasonography-guided in-plane method, bilateral proximal GON block was performed, between the inferior oblique capitis and splenius capitis muscles. The injected materials were a combination of lidocaine and bupivacaine at concentrations of 0.5% and 0.25%, respectively. A total of 2 mL of the mixed solution was injected at each site.
3. Follow-up and evaluation
The patients were followed up with 4-week intervals and instructed to keep a conventional headache diary every day during the treatment period. The diaries were reviewed on every follow-up appointment. Every patient were injected and evaluated for two sessions.
4. Wear-off phenomenon
Wear-off phenomenon was monitored between the day of bilateral GON block and the day of next onabotulinumtoxinA injection. The wear-off event was defined as a reduction in headache at week 8 (during 1 week before nerve block) followed by a worsening of headache at week 9–12, before the next onabobulinumtoxinA injection. This suggests that the initial reduction in headache at week 8 is associated with the effect of onabotulinumtoxinA injection, while a subsequent worsening of headache, despite nerve block treatment, indicates a wear-off phenomenon.
RESULTS
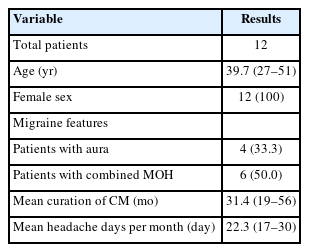
We reviewed 12 patients who received onabotulinumtoxinA and nerve block for CM at a single center, under the care of a single neurologist, from January 2023 to December 2023. Demographics of patients are summarized in Table 1 and baseline characteristics and results are summarized in Table 2.
All the patients were female, with a mean age of 39.7 years (range, 27 to 51 years). Four patients (33.3%) presented with aura alongside CM, and six patients (50.0%) were diagnosed with medication-overuse headache. The mean duration of CM was 31.4 months (range, 19 to 56 months). On average, patients experienced 22.3 headache days per month (range, 17 to 30 days).
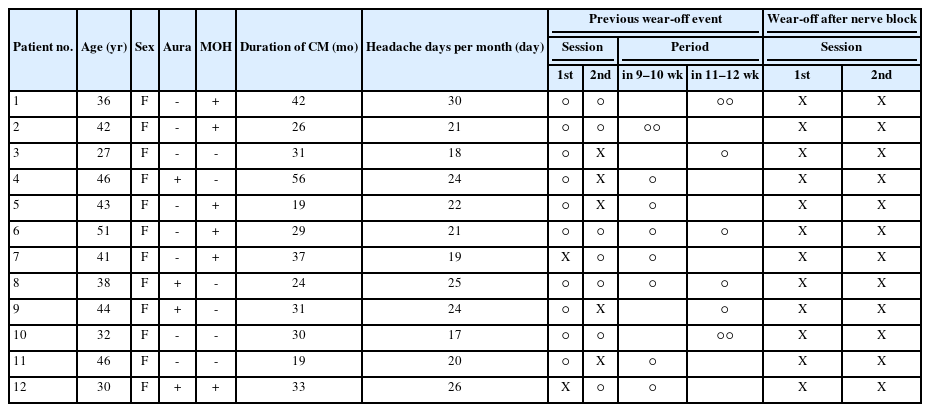
All patients experienced a wear-off phenomenon during previous onabotulinumtoxinA treatment. Specifically, 10 patients (83.3%) exhibited the wear-off phenomenon during the previous first injection session, while seven patients (58.3%) experienced it during the previous second injection session. Additionally, five patients (41.7%) encountered the wear-off phenomenon during both the previous first and second sessions.
The starting period of the previous wear-off phenomenon was also assessed. Out of a total of 17 events, eight events (47.1%) occurred after 10 weeks from the onabotulinumtoxinA injection, and nine events (52.9%) occurred during 9 and 10 weeks from the onabotulinumtoxinA injection.
The wear-off phenomenon was monitored following an additional ultrasonography-guided bilateral GON block, conducted after 8 weeks of the each first and second onabotulinumtoxinA injection. The results revealed no occurrence (0%) of the wear-off phenomenon among any of the patients who experienced the previous wear-off.
DISCUSSION
The wear-off phenomenon observed in CM patients undergoing onabotulinumtoxinA treatment presents a significant challenge in long-term management. In this study, we explored the potential of bilateral GON block as a preventive strategy to alleviate wear-off events. Our findings suggest that the addition of GON block to the treatment regimen effectively prevents wear-off phenomenon in patients with CM.
In our knowledge, there are no detailed previous study about prevention for wear-off phenomenon, especially regarding peripheral nerve block. One study showed that one of the most common therapies used during wear-off phenomenon is peripheral nerve block. However, it was not about preventive treatment, but rather about treatment after the wear-off had occurred. Furthermore, the specific method of peripheral nerve block including material, dose, timing and region was not detailed.8 The definition of wear-off phenomenon used for patient enrollment in this study was worsening of headache at week 9–12. Therefore, in this study, considering the immediate effect of the peripheral nerve block, we performed nerve block exactly 8 weeks after the first and second sessions of onabotulinumtoxinA injection. It is known that the effect of a single GON block can last up to a month, far longer than its anesthetic effect.12 Moreover, a recent study revealed that monthly GON block was effective in reducing migraine days in CM.11 Therefore, it was expected that a single GON block in CM would show an immediate and sustained effect throughout the wear-off phenomenon period of about a month. In addition, although the academic evidence of GON block in CM has been accumulated to some extent, the evidence of peripheral nerve block in other region is still deficient. Therefore our study performed only GON block excluding other peripheral nerve blocks. A study by Abbas et al.13 demonstrated that bilateral ultrasound-guided GON block reduced interictal CGRP level in CM. Additionally, research by Karaoğlan14 showed that the combination of onabotulinumtoxinA injection and ultrasound-guided bilateral proximal GON block was more effective than onabotulinumtoxinA monotherapy. Therefore, in this study, ultrasound-guided bilateral proximal GON block was planned to guarantee maximal effects of peripheral nerve block in CM.
First of all, our results demonstrate that ultrasound-guided bilateral proximal GON block administered at 8 weeks after the initial onabotulinumtoxinA injection effectively prevents wear-off events. From previous studies, it has been reported that the wear-off phenomenon is common among patients receiving onabotulinumtoxinA.8,15 Despite several studies conducted to identify risk factors for the wear-off phenomenon, no definitive factors have been discovered.5 Consequently, it is challenging to predict which patients will require additional strategies to avoid the wear-off phenomenon. Nonetheless, all the patients in our study had experienced wear-off on previous onabotulinumtoxinA treatment, which means they could have been susceptible to wear-off phenomenon. After additional treatment with GON block, none of the patients experienced wear-off phenomenon. This highlights the potential of GON block as a preventive strategy to sustain the therapeutic benefits of onabotulinumtoxinA over the treatment interval.
The underlying mechanism of the wear-off phenomenon remains unclear,4 but it is hypothesized to be related to the diminishing effectiveness of onabotulinumtoxinA over time.7,16 Mechanisms of action of GON block in CM are also not fully known. GON blocks, which provide regional anesthesia, may offer prolonged relief by breaking the pain cycle involved in central and peripheral sensitization.17 The effect of GON block has been proposed to be due to a modulatory effect of GON block through trigeminocervical complex to the higher pain pathway.18 Moreover, a recent study showed that GON block significantly reduced interictal CGRP level in CM.13 Elevated serum CGRP has been suggested as a biomarker to facilitate a more objective diagnosis of CM.19 These findings led to a better understanding of GON block’s mode of action in CM. The combination of onabotulinumtoxinA and GON blocks could therefore provide a synergistic effect, enhancing overall treatment efficacy.
It is important to note that both onabotulinumtoxinA injections and GON blocks are procedural interventions performed by physicians. Therefore, differences in procedural skill and technique may influence treatment outcomes. In this study, we minimized procedure-related errors by performing ultrasound-guided in-plane GON blocks, allowing more precise injection into the targeted nerve. Furthermore, all GON block and onabolulinumtoxinA procedures were performed by a single skilled and experienced professional physician.
The clinical implications of our study are profound. As mentioned above, addition of GON blocks can be a viable strategy to prevent the wear-off phenomenon and maintain continuous headache relief in CM patients. This approach can potentially improve patient adherence to the treatment and overall quality of life by reducing headache severity and frequency.
Despite the promising findings of our study, several limitations should be acknowledged. The small number of patients and sessions, as well as the retrospective nature of our analysis, may limit the generalizability of our results. Additionally, the lack of a control group and the absence of long-term follow-up data preclude definitive conclusions regarding the sustained efficacy of GON block in preventing wear-off phenomenon. The placebo effect of the procedure is known to be particularly large in pain research, including headaches. In addition, it should be taken into account that the group that participated in this study were patients who had previously experienced troublesome wear-off, so selection bias and placebo effects may be greater. Also, various trials should be made to determine how often and when prophylactic nerve blocks with different techniques should be performed to prevent the wear-off phenomenon of onabotulinumtoxinA injection.
In conclusion, our study suggests that bilateral GON block may serve as an effective preventive strategy for alleviating wear-off phenomenon in CM patients undergoing onabotulinumtoxinA treatment. Further research incorporating larger patient cohorts and longer follow-up periods is warranted to validate these findings and optimize the clinical management of CM.
Notes
AVAILABILITY OF DATA AND MATERIAL
The data presented in this study are available upon reasonable request from the corresponding author.
AUTHOR CONTRIBUTIONS
Conceptualization: JYA, DWB; Data curation: JYA, DWB; Methodology: DWB; Supervision: JYA; Writing–original draft: SK; Writing–review & editing: SK, JYA, DWB.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING STATEMENT
Not applicable.
ACKNOWLEDGMENTS
Not applicable.