Articles
- Page Path
- HOME > Headache Pain Res > Volume 26(3); 2025 > Article
-
Review Article
A Practical Approach to Headache in Moyamoya Disease -
Mi-Yeon Eun1,2
 , Jin-Man Jung3
, Jin-Man Jung3 , Jay Chol Choi4
, Jay Chol Choi4
-
Headache and Pain Research 2025;26(3):173-183.
DOI: https://doi.org/10.62087/hpr.2025.0011
Published online: October 17, 2025
1Department of Neurology, School of Medicine, Kyungpook National University, Daegu, Republic of Korea
2Department of Neurology, Kyungpook National University Chilgok Hospital, Daegu, Republic of Korea
3Department of Neurology, Korea University Ansan Hospital, Ansan, Republic of Korea
4Department of Neurology, Jeju National University Hospital, Jeju National University College of Medicine, Jeju, Republic of Korea
- Correspondence: Jay Chol Choi, M.D., Ph.D. Department of Neurology, Jeju National University College of Medicine, 15 Aran 13-gil, Jeju 63241, Republic of Korea Tel: +82-64-754-8160, Fax: +82-64-717-1131, E-mail: jaychoi@jejunu.ac.kr
© 2025 The Korean Headache Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 942 Views
- 15 Download
- 1 Crossref
Abstract
- Moyamoya disease (MMD) is a progressive steno-occlusive cerebrovascular disorder of the intracranial internal carotid arteries characterized by fragile collateral vessel formation. Although ischemic and hemorrhagic strokes are the most widely recognized manifestations of MMD, headaches are common, often disabling, and remain underacknowledged. Epidemiological studies report headache in 17%–85% of MMD patients, with particularly high rates among pediatric patients. Clinically, headache phenotypes are diverse and include migraine-like headaches with or without aura, tension-type, cluster, and hemiplegic variants. These presentations often overlap with primary headache disorders, complicating the diagnosis and sometimes delaying the recognition of underlying MMD. The pathophysiology of MMD-related headaches is multifactorial, involving vascular stenosis, abnormal collateral circulation, altered hemodynamics, and neurogenic inflammation. Chronic hypoperfusion may lower the threshold for cortical spreading depression, contributing to migraine-like or aura-associated symptoms. Surgical revascularization has been reported to alleviate headaches in both pediatric and adult patients, but persistent or new headaches may occur postoperatively, and long-term outcomes remain inconsistent. Management often involves general analgesics such as acetaminophen and non-steroidal anti-inflammatory drugs, but vasoconstrictive agents (e.g., triptans and ergotamines) should be avoided. Lasmiditan, a non-vasoconstrictive 5-HT1F agonist, may represent a safer option for acute treatment, while the efficacy of other pharmacological agents remains unclear due to limited evidence. In conclusion, headaches in MMD are not only a frequent source of disability but also a potential clinical marker of disease activity. Wider recognition of their epidemiology, phenotypes, and mechanisms may improve the diagnosis, guide individualized treatment, and ultimately enhance quality of life for patients.
INTRODUCTION
EPIDEMIOLOGY OF HEADACHES IN MOYAMOYA DISEASE
CHARACTERISTICS OF HEADACHE IN MOYAMOYA DISEASE
PATHOPHYSIOLOGICAL MECHANISMS OF HEADACHES IN MOYAMOYA DISEASE
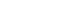
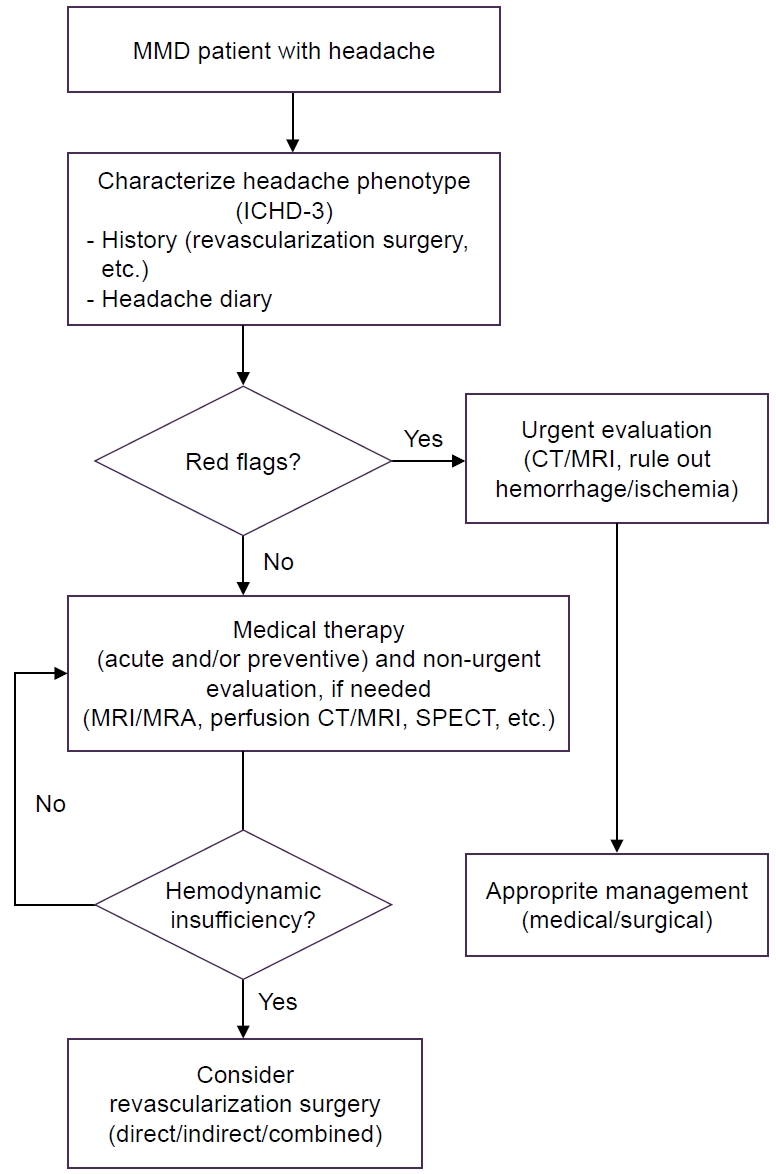
MANAGEMENT OF HEADACHES IN MOYAMOYA DISEASE
BROADER IMPLICATIONS, APPLICATIONS, AND CONCLUSIONS
AVAILABILITY OF DATA AND MATERIAL
The data presented in this study are available upon reasonable request from the corresponding author.
AUTHOR CONTRIBUTIONS
Conceptualization: JCC; Writing–original draft: MYE, JMJ, JCC; Writing–review & editing: MYE, JMJ, JCC.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING STATEMENT
Not applicable.
ACKNOWLEDGMENTS
Not applicable.
| Author (year) | Country | Study design | Total patients (n) | Age (yr) | Study population | Headache, n (%) | Migraine, n (%) | Migraine with aura, n (%) | Tension-type, n (%) | Other headache type, n (%) | Headache severity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Matsushima et al. (1990)13 | Japan | Retrospective | 81 | 10.4 | Pediatric | 22 (27.2) | N/A | N/A | N/A | N/A | N/A |
| Yu et al. (1991)16 | Korea | Retrospective | 38 | 6.3, 36.8† | Both | 10 (26.3) | N/A | N/A | N/A | N/A | N/A |
| Battistella and Carollo (1997)24 | Italy | Retrospective | 34 | 13.5 | Both | 7 (20.6) | 7 (100) | N/A | 0 | 0 | N/A |
| Seol et al. (2005)8 | Korea | Retrospective | 204 | 7 | Pediatric | 44 (21.6) | N/A | N/A | N/A | Morning HA 14 (31.8) | N/A |
| Kim et al. (2010)9 | Korea | Retrospective | 410 | 7.3 | Pediatric | 134 (32.7) | N/A | N/A | N/A | N/A | Severe headache |
| Okada et al. (2012)12 | Japan | Retrospective | 117 | 33 | Both | 25 (21.4) | N/A | N/A | N/A | N/A | Grade 1* 4 (16.0), Grade 2 13 (52.0), Grade 3 8/25 (32.0) |
| Hoshino et al. (2012)25 | Japan | Retrospective | 941 | N/A | Both | 56 (6.0) | N/A | N/A | N/A | N/A | N/A |
| Kawabori et al. (2013)10 | Japan | Retrospective | 29 | 10 | Pediatric | 11 (37.9) | N/A | N/A | N/A | N/A | Severe |
| Amlie-Lefond and Ellenbogen (2015)20 | USA | Retrospective | 54 | 7.5 | Pediatric | 28 (51.9) | 6 (21.4) | 1 (16.7%, hemiplegic migraine) | N/A | N/A | N/A |
| Bohara et al. (2015)14 | Japan | Retrospective | 10 | 8 | Pediatric | 8 (80.0) | N/A | N/A | N/A | N/A | N/A |
| Kraemer et al. (2017)17 | Germany | Retrospective | 55 | 37.6 | Adult | 37 (67.3) | 17 (45.9) | 10 (27.0) | 10 (27.0) | Mixed 10 (27.0; migraine+TTHA) | NRS mean 3.2±1.3 |
| Tho-Calvi et al. (2018)19 | UK | Retrospective | 88 | 5.1 | Pediatric | 50 (56.8) | N/A | N/A | N/A | N/A | N/A |
| Katano et al. (2019)15 | Japan | Retrospective | 68 | 49.8 | Adult | 33 (48.5) | N/A | N/A | N/A | N/A | Light 19 (57.6), moderate 12 (36.4), severe 3/33 (9.1) |
| Gao et al. (2020)23 | China | Retrospective | 119 | 34.4 | Both | 119 (100) | 46 (38.7) | 34 (73.9) | 29 (24.4) | Mixed 44 (37.0; migraine+TTHA) | Severe 67 (56.3), VAS 5.9 |
| Das et al. (2020)22 | India | Retrospective | 76 | 21.2 | Both | 28 (36.8) | N/A | N/A | N/A | N/A | N/A |
| Aihara et al. (2021)7 | Japan | Retrospective | 35 | 10.5 | Pediatric | 29/34 (85.3) | N/A | N/A | N/A | N/A | Moderate 9 (25.7), intractable 20 (57.1) |
| Sutton et al. (2022)26 | USA | Retrospective | 12 | 42 | Adult | 4 (33.3) | 4 (100) | N/A | N/A | N/A | N/A |
| Das et al. (2022)27 | India | Retrospective | 6 | 23.7 | Both | 3 (50.0) | N/A | N/A | 2 (33.3) | N/A | Mild to moderate |
| Teo et al. (2022)18 | USA | Retrospective | 769 | 32 | Both | 354 (46.0) | N/A | N/A | N/A | N/A | N/A |
| Vassilopoulou et al. (2023)11 | Greece | Prospective | 12 | 43.5 | Adult | 2 (16.7) | N/A | N/A | N/A | N/A | N/A |
| Gallego Moyano et al. (2024)21 | Canada | Retrospective | 59 | 47 | Adult | 41 (69.5) | N/A | N/A | N/A | N/A | VAS median 5 |
N/A, not available; Morning HA, morcing headache; TTHA, tension-type headache; NRS, Numerical Rating Scale; VAS, visual analogue scale.
*Grade 1, severe headache not requiring medication and/or rest; Grade 2, severe headache requiring medication (non-steroidal anti-inflammatory drugs and/or medication for migraine); and Grade 3, severe headache requiring medication and rest. †Mean age of pediatric and adult patients, respectively.
| Author (year) | Sex | Age (yr) | MMD | Headache type | Location | Severity | Combined symptoms | Neurological symptoms | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Park-Matsumoto et al. (1999)34 | Female | 49 | Bilateral | Migraine with aura | N/A | Severe | N/A | Cerebral infarction | N/A | Gradually improved, no recurrence |
| Aydin et al. (2003)35 | Female | 4 | Bilateral | Migraine | N/A | Severe | Nausea, vomiting, photophobia | None | Nimodipine | Improved headache |
| Sewell et al. (2009)28 | Male | 34 | Bilateral | Cluster headache | N/A | Severe | Rhinorrhea, lacrimation, ptosis | Left-sided RLS, left hand numbness | Revascularization | Improved after revascularization |
| Siddiqui et al. (2010)29 | Female | 10 | Bilateral | Hemiplegic migraine | Left hemicranial | Moderate | Nausea, vomiting, photophobia | Left hemiparesis | NSAIDs, propranolol, prochlorperazine, nimodipine, aspirin | Gradually improved |
| Zach et al. (2010)5 | Female | 39 | Bilateral | Migraine without aura, tension-type headache | Bilateral frontal | Moderate to severe | Photophobia, allodynia, nausea, light headedness | Tinnitus | NSAIDs, acetaminophen | N/A |
| Verdure et al. (2012)31 | Female | 13 | Bilateral | Migraine without aura | N/A | N/A | N/A | TIA, left ACA territory infarction | N/A | N/A |
| Vuignier et al. (2014)32 | Female | 4 | Right | N/A | Right frontal | N/A | Vomiting | Ischemic stroke | Revascularization | Improved headache after revascularization |
| Lee et al. (2014)36 | Female | 12 | Bilateral | N/A | Bitemporal | Severe | Eyeball pain | TIA, dysarthria, right hemiparesis, numbness | NSAIDs, topiramate | Not effective |
| Diaz et al. (2014)37 | Female | 32 | Left | Migraine | N/A | Intractable | Nausea, vomiting | N/A | Aspirin | N/A |
| Tozzi et al. (2015)30 | Female | 7 | Bilateral | N/A | Frontal | N/A | N/A | Drop attacks, dysarthria, chorea, hemiparesis | Revascularization | Improved headache intensity and frequency (1 time/wk) |
| Khan et al. (2025)33 | Male | 65 | Right | N/A | Right-sided | Severe | N/A | Left-sided numbness | Aspirin | Improved |
- 1. Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol 1969;20:288-299.ArticlePubMed
- 2. Kamada F, Aoki Y, Narisawa A, et al. A genome-wide association study identifies RNF213 as the first Moyamoya disease gene. J Hum Genet 2011;56:34-40.ArticlePubMedPDF
- 3. Milewicz DM, Østergaard JR, Ala-Kokko LM, et al. De novo ACTA2 mutation causes a novel syndrome of multisystemic smooth muscle dysfunction. Am J Med Genet A 2010;152A:2437-2443.ArticlePubMedPMC
- 4. Hervé D, Philippi A, Belbouab R, et al. Loss of α1β1 soluble guanylate cyclase, the major nitric oxide receptor, leads to moyamoya and achalasia. Am J Hum Genet 2014;94:385-394.ArticlePubMedPMC
- 5. Zach V, Bezov D, Lipton RB, Ashina S. Headache associated with moyamoya disease: a case story and literature review. J Headache Pain 2010;11:79-82.ArticlePubMedPMCPDF
- 6. Chiang CC, Shahid AH, Harriott AM, et al. Evaluation and treatment of headache associated with moyamoya disease: a narrative review. Cephalalgia 2022;42:542-552.ArticlePubMedPDF
- 7. Aihara Y, Kashiwase S, Chiba K, et al. Aspirin use and platelet aggregation in ischemic onset-type pediatric moyamoya patients with intractable headaches (moya-ache). Childs Nerv Syst 2021;37:1649-1657.ArticlePubMedPDF
- 8. Seol HJ, Wang KC, Kim SK, Hwang YS, Kim KJ, Cho BK. Headache in pediatric moyamoya disease: review of 204 consecutive cases. J Neurosurg 2005;103:439-442.ArticlePubMed
- 9. Kim SK, Cho BK, Phi JH, et al. Pediatric moyamoya disease: an analysis of 410 consecutive cases. Ann Neurol 2010;68:92-101.ArticlePubMed
- 10. Kawabori M, Kuroda S, Nakayama N, et al. Effective surgical revascularization improves cerebral hemodynamics and resolves headache in pediatric Moyamoya disease. World Neurosurg 2013;80:612-619.ArticlePubMed
- 11. Vassilopoulou S, Tountopoulou A, Korompoki E, et al. Moyamoya disease: clinical and radiological characteristics in adult Greek patients. J Clin Med 2023;12:5951.ArticlePubMedPMC
- 12. Okada Y, Kawamata T, Kawashima A, Yamaguchi K, Ono Y, Hori T. The efficacy of superficial temporal artery-middle cerebral artery anastomosis in patients with moyamoya disease complaining of severe headache. J Neurosurg 2012;116:672-679.ArticlePubMed
- 13. Matsushima Y, Aoyagi M, Niimi Y, Masaoka H, Ohno K. Symptoms and their pattern of progression in childhood moyamoya disease. Brain Dev 1990;12:784-789.ArticlePubMed
- 14. Bohara M, Sugata S, Nishimuta Y, et al. Effect of revascularization on headache associated with moyamoya disease in pediatric patients. Hiroshima J Med Sci 2015;64:39-44.PubMed
- 15. Katano H, Nishikawa Y, Yamada H, Shibata T, Miyachi S, Mase M. Association of superficial temporal artery dilatation with headache after revascularization in adult moyamoya disease. World Neurosurg 2019;129:e594-e606.ArticlePubMed
- 16. Yu GJ, Kim SY, Coe CJ. Moyamoya disease in Korea. Yonsei Med J 1991;32:263-269.ArticlePubMed
- 17. Kraemer M, Lee SI, Ayzenberg I, et al. Headache in Caucasian patients with Moyamoya angiopathy: a systematic cohort study. Cephalalgia 2017;37:496-500.ArticlePubMedPDF
- 18. Teo M, Abhinav K, Bell-Stephens TE, et al. Short- and long-term outcomes of moyamoya patients post-revascularization. J Neurosurg 2022;138:1374-1384.ArticlePubMed
- 19. Tho-Calvi SC, Thompson D, Saunders D, et al. Clinical features, course, and outcomes of a UK cohort of pediatric moyamoya. Neurology 2018;90:e763-e770.ArticlePubMed
- 20. Amlie-Lefond C, Ellenbogen RG. Factors associated with the presentation of moyamoya in childhood. J Stroke Cerebrovasc Dis 2015;24:1204-1210.ArticlePubMed
- 21. Gallego Moyano FD, Janssen HC, Venkatraghavan L, et al. The impact of revascularization surgery on headaches in association with cerebrovascular reactivity in patients with Moyamoya angiopathy. Brain Sci 2024;14:967.ArticlePubMedPMC
- 22. Das S, Dubey S, Acharya M, et al. The disease presentation of Moyamoya angiopathy in Eastern India. J Stroke Cerebrovasc Dis 2020;29:104957.ArticlePubMed
- 23. Gao B, Kang K, Zhang J, Zhang D, Zhao X. Clinical characteristics and long-term outcome of headaches associated with Moyamoya disease in the Chinese population: a cohort study. Front Neurol 2020;11:605636.ArticlePubMedPMC
- 24. Battistella PA, Carollo C. Clinical and neuroradiological findings of moyamoya disease in Italy. Clin Neurol Neurosurg 1997;99 Suppl 2:S54-S57.ArticlePubMed
- 25. Hoshino H, Izawa Y, Suzuki N; Research Committee on Moyamoya Disease. Epidemiological features of moyamoya disease in Japan. Neurol Med Chir (Tokyo) 2012;52:295-298.ArticlePubMed
- 26. Sutton CXY, Carrazana E, Mitchell C, Viereck J, Liow KK, Ghaffari-Rafi A. Identification of associations and distinguishing moyamoya disease from ischemic strokes of other etiologies: a retrospective case-control study. Ann Med Surg (Lond) 2022;78:103771.ArticlePubMedPMC
- 27. Das S, Ray BK, Ghosh R, Dubey S. “Asymptomatic” Moyamoya Angiopathy: is it truly asymptomatic? J Stroke Cerebrovasc Dis 2022;31:106432.ArticlePubMed
- 28. Sewell RA, Johnson DJ, Fellows DW. Cluster headache associated with moyamoya. J Headache Pain 2009;10:65-67.ArticlePubMedPMCPDF
- 29. Siddiqui MR, Khan SU, Hoque MA, Rahman KM, Mondol MB, Mohammad QD. Moyamoya disease presented as a case of hemiplegic migraine. BMJ Case Rep 2010;2010:bcr0220102764.ArticlePubMedPMC
- 30. Tozzi E, Antenucci A, Di Loreto S, Maresca M, Farello G, Massimi L. O063. Moyamoya disease and headache: case report. J Headache Pain 2015;16:A73.ArticlePubMedPMCPDF
- 31. Verdure P, Le Moigne O, Massardier EG, Vanhulle C, Tollard E, Maltête D. Migraine-like headache and ocular malformations may herald Moyamoya syndrome. Rev Neurol (Paris) 2012;168:460-461.ArticlePubMed
- 32. Vuignier S, Akioka N, Hamada H, Kashiwazaki D, Kuroda S. Headache attack followed by rapid disease progression in pediatric moyamoya disease: how should we manage it? Childs Nerv Syst 2014;30:1733-1736.ArticlePubMedPDF
- 33. Khan F, Fatima F, Malik F, et al. Atypical presentation of moyamoya disease presenting with severe headache: a case report. Cureus 2025;17:e80378.ArticlePubMedPMC
- 34. Park-Matsumoto YC, Tazawa T, Shimizu J. Migraine with aura-like headache associated with moyamoya disease. Acta Neurol Scand 1999;100:119-121.ArticlePubMed
- 35. Aydin K, Okuyaz C, Gücüyener K, Serdaroğlu A, Akpek S. Moyamoya disease presented with migrainelike headache in a 4-year-old girl. J Child Neurol 2003;18:361-363.ArticlePubMedPDF
- 36. Lee WJ, Kim YO, Yoon W, Rho YI, Woo YJ. Headache associated with moyamoya disease in a child: pain pattern in MRA progression. J Korean Child Neurol Soc 2014;22:98-101.Article
- 37. Diaz UJ, Cabán-Martinez AJ, Halder GE. Presentation with recurrent intractable headache: a patient with moyamoya syndrome: case report. Neurol Med Chir (Tokyo) 2014;54:133-135.ArticlePubMedPMC
- 38. Uchino H, Kazumata K, Ito M, Nakayama N, Houkin K. Novel insights into symptomatology of moyamoya disease in pediatric patients: survey of symptoms suggestive of orthostatic intolerance. J Neurosurg Pediatr 2017;20:485-488.ArticlePubMed
- 39. Feng Z, Chang Y, Fu C. Ruptured peripheral cerebral aneurysms associated with moyamoya disease: a systematic review. J Stroke 2024;26:360-370.ArticlePubMedPMCPDF
- 40. Holst H, Arendt-Nielsen L, Mosbech H, Serup J, Elberling J. Capsaicin-induced neurogenic inflammation in the skin in patients with symptoms induced by odorous chemicals. Skin Res Technol 2011;17:82-90.ArticlePubMed
- 41. Buzzi MG, Moskowitz MA. The trigemino-vascular system and migraine. Pathol Biol (Paris) 1992;40:313-317.PubMed
- 42. Khan S, Amin FM, Christensen CE, et al. Meningeal contribution to migraine pain: a magnetic resonance angiography study. Brain 2019;142:93-102.ArticlePubMed
- 43. Spekker E, Tanaka M, Szabó Á, Vécsei L. Neurogenic inflammation: the participant in migraine and recent advancements in translational research. Biomedicines 2021;10:76.ArticlePubMedPMC
- 44. Olesen J, Friberg L, Olsen TS, et al. Ischaemia-induced (symptomatic) migraine attacks may be more frequent than migraine-induced ischaemic insults. Brain 1993;116:187-202.ArticlePubMed
- 45. Menyhárt Á, Varga DP, M Tóth O, Makra P, Bari F, Farkas E. Transient hypoperfusion to ischemic/anoxic spreading depolarization is related to autoregulatory failure in the rat cerebral cortex. Neurocrit Care 2022;37:112-122.ArticlePubMedPMCPDF
- 46. Konsman JP, Drukarch B, Van Dam AM. (Peri)vascular production and action of pro-inflammatory cytokines in brain pathology. Clin Sci (Lond) 2007;112:1-25.ArticlePubMedPDF
- 47. Canavero I, Vetrano IG, Zedde M, et al. Clinical management of moyamoya patients. J Clin Med 2021;10:3628.ArticlePubMedPMC
- 48. Shapiro RE, Hochstetler HM, Dennehy EB, et al. Lasmiditan for acute treatment of migraine in patients with cardiovascular risk factors: post-hoc analysis of pooled results from 2 randomized, double-blind, placebo-controlled, phase 3 trials. J Headache Pain 2019;20:90.ArticlePubMedPMCPDF
- 49. Nelson DL, Phebus LA, Johnson KW, et al. Preclinical pharmacological profile of the selective 5-HT1F receptor agonist lasmiditan. Cephalalgia 2010;30:1159-1169.ArticlePubMedPDF
- 50. Favoni V, Giani L, Al-Hassany L, et al. CGRP and migraine from a cardiovascular point of view: what do we expect from blocking CGRP? J Headache Pain 2019;20:27.ArticlePubMedPMC
- 51. Zhai L, Sakurai T, Kamiyoshi A, et al. Endogenous calcitonin gene-related peptide suppresses ischemic brain injuries and progression of cognitive decline. J Hypertens 2018;36:876-891.ArticlePubMed
- 52. Seo WK, Kim JY, Choi EH, et al. Association of antiplatelet therapy, including cilostazol, with improved survival in patients with moyamoya disease in a nationwide study. J Am Heart Assoc 2021;10:e017701.ArticlePubMedPMC
- 53. Kim JY, Kim HJ, Choi EH, et al. Vessel wall changes on serial high-resolution MRI and the use of cilostazol in patients with adult-onset moyamoya disease. J Clin Neurol 2022;18:610-618.ArticlePubMedPMCPDF
- 54. Kim BS, Chung PW, Chung JM, et al. Evidence-based recommendations on pharmacologic treatment for migraine prevention: a clinical practice guideline from the Korean Headache Society. Headache Pain Res 2025;26:5-20.ArticlePDF
- 55. Bidaki R, Zarepur E. Intermittent hemiplegia in a boy with primary moyamoya disease: a case report from Iran. Iran J Child Neurol 2017;11:65-68.Article
REFERENCES
Figure & Data
References
Citations

- Characteristics of sleep disturbance in Moyamoya disease
Mudassar Ahmad, James P. Klaas, Giuseppe Lanzino, Lorenzo Rinaldo, Stuart J. McCarter, Virend K. Somers, Erik K. St Louis, Diego Z. Carvalho
Sleep Medicine.2026; 138: 108684. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
- Related articles
Figure 1.
| Author (year) | Country | Study design | Total patients (n) | Age (yr) | Study population | Headache, n (%) | Migraine, n (%) | Migraine with aura, n (%) | Tension-type, n (%) | Other headache type, n (%) | Headache severity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Matsushima et al. (1990)13 | Japan | Retrospective | 81 | 10.4 | Pediatric | 22 (27.2) | N/A | N/A | N/A | N/A | N/A |
| Yu et al. (1991)16 | Korea | Retrospective | 38 | 6.3, 36.8† | Both | 10 (26.3) | N/A | N/A | N/A | N/A | N/A |
| Battistella and Carollo (1997)24 | Italy | Retrospective | 34 | 13.5 | Both | 7 (20.6) | 7 (100) | N/A | 0 | 0 | N/A |
| Seol et al. (2005)8 | Korea | Retrospective | 204 | 7 | Pediatric | 44 (21.6) | N/A | N/A | N/A | Morning HA 14 (31.8) | N/A |
| Kim et al. (2010)9 | Korea | Retrospective | 410 | 7.3 | Pediatric | 134 (32.7) | N/A | N/A | N/A | N/A | Severe headache |
| Okada et al. (2012)12 | Japan | Retrospective | 117 | 33 | Both | 25 (21.4) | N/A | N/A | N/A | N/A | Grade 1* 4 (16.0), Grade 2 13 (52.0), Grade 3 8/25 (32.0) |
| Hoshino et al. (2012)25 | Japan | Retrospective | 941 | N/A | Both | 56 (6.0) | N/A | N/A | N/A | N/A | N/A |
| Kawabori et al. (2013)10 | Japan | Retrospective | 29 | 10 | Pediatric | 11 (37.9) | N/A | N/A | N/A | N/A | Severe |
| Amlie-Lefond and Ellenbogen (2015)20 | USA | Retrospective | 54 | 7.5 | Pediatric | 28 (51.9) | 6 (21.4) | 1 (16.7%, hemiplegic migraine) | N/A | N/A | N/A |
| Bohara et al. (2015)14 | Japan | Retrospective | 10 | 8 | Pediatric | 8 (80.0) | N/A | N/A | N/A | N/A | N/A |
| Kraemer et al. (2017)17 | Germany | Retrospective | 55 | 37.6 | Adult | 37 (67.3) | 17 (45.9) | 10 (27.0) | 10 (27.0) | Mixed 10 (27.0; migraine+TTHA) | NRS mean 3.2±1.3 |
| Tho-Calvi et al. (2018)19 | UK | Retrospective | 88 | 5.1 | Pediatric | 50 (56.8) | N/A | N/A | N/A | N/A | N/A |
| Katano et al. (2019)15 | Japan | Retrospective | 68 | 49.8 | Adult | 33 (48.5) | N/A | N/A | N/A | N/A | Light 19 (57.6), moderate 12 (36.4), severe 3/33 (9.1) |
| Gao et al. (2020)23 | China | Retrospective | 119 | 34.4 | Both | 119 (100) | 46 (38.7) | 34 (73.9) | 29 (24.4) | Mixed 44 (37.0; migraine+TTHA) | Severe 67 (56.3), VAS 5.9 |
| Das et al. (2020)22 | India | Retrospective | 76 | 21.2 | Both | 28 (36.8) | N/A | N/A | N/A | N/A | N/A |
| Aihara et al. (2021)7 | Japan | Retrospective | 35 | 10.5 | Pediatric | 29/34 (85.3) | N/A | N/A | N/A | N/A | Moderate 9 (25.7), intractable 20 (57.1) |
| Sutton et al. (2022)26 | USA | Retrospective | 12 | 42 | Adult | 4 (33.3) | 4 (100) | N/A | N/A | N/A | N/A |
| Das et al. (2022)27 | India | Retrospective | 6 | 23.7 | Both | 3 (50.0) | N/A | N/A | 2 (33.3) | N/A | Mild to moderate |
| Teo et al. (2022)18 | USA | Retrospective | 769 | 32 | Both | 354 (46.0) | N/A | N/A | N/A | N/A | N/A |
| Vassilopoulou et al. (2023)11 | Greece | Prospective | 12 | 43.5 | Adult | 2 (16.7) | N/A | N/A | N/A | N/A | N/A |
| Gallego Moyano et al. (2024)21 | Canada | Retrospective | 59 | 47 | Adult | 41 (69.5) | N/A | N/A | N/A | N/A | VAS median 5 |
| Author (year) | Sex | Age (yr) | MMD | Headache type | Location | Severity | Combined symptoms | Neurological symptoms | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Park-Matsumoto et al. (1999)34 | Female | 49 | Bilateral | Migraine with aura | N/A | Severe | N/A | Cerebral infarction | N/A | Gradually improved, no recurrence |
| Aydin et al. (2003)35 | Female | 4 | Bilateral | Migraine | N/A | Severe | Nausea, vomiting, photophobia | None | Nimodipine | Improved headache |
| Sewell et al. (2009)28 | Male | 34 | Bilateral | Cluster headache | N/A | Severe | Rhinorrhea, lacrimation, ptosis | Left-sided RLS, left hand numbness | Revascularization | Improved after revascularization |
| Siddiqui et al. (2010)29 | Female | 10 | Bilateral | Hemiplegic migraine | Left hemicranial | Moderate | Nausea, vomiting, photophobia | Left hemiparesis | NSAIDs, propranolol, prochlorperazine, nimodipine, aspirin | Gradually improved |
| Zach et al. (2010)5 | Female | 39 | Bilateral | Migraine without aura, tension-type headache | Bilateral frontal | Moderate to severe | Photophobia, allodynia, nausea, light headedness | Tinnitus | NSAIDs, acetaminophen | N/A |
| Verdure et al. (2012)31 | Female | 13 | Bilateral | Migraine without aura | N/A | N/A | N/A | TIA, left ACA territory infarction | N/A | N/A |
| Vuignier et al. (2014)32 | Female | 4 | Right | N/A | Right frontal | N/A | Vomiting | Ischemic stroke | Revascularization | Improved headache after revascularization |
| Lee et al. (2014)36 | Female | 12 | Bilateral | N/A | Bitemporal | Severe | Eyeball pain | TIA, dysarthria, right hemiparesis, numbness | NSAIDs, topiramate | Not effective |
| Diaz et al. (2014)37 | Female | 32 | Left | Migraine | N/A | Intractable | Nausea, vomiting | N/A | Aspirin | N/A |
| Tozzi et al. (2015)30 | Female | 7 | Bilateral | N/A | Frontal | N/A | N/A | Drop attacks, dysarthria, chorea, hemiparesis | Revascularization | Improved headache intensity and frequency (1 time/wk) |
| Khan et al. (2025)33 | Male | 65 | Right | N/A | Right-sided | Severe | N/A | Left-sided numbness | Aspirin | Improved |
N/A, not available; Morning HA, morcing headache; TTHA, tension-type headache; NRS, Numerical Rating Scale; VAS, visual analogue scale. *Grade 1, severe headache not requiring medication and/or rest; Grade 2, severe headache requiring medication (non-steroidal anti-inflammatory drugs and/or medication for migraine); and Grade 3, severe headache requiring medication and rest. †Mean age of pediatric and adult patients, respectively.
MMD, Moyamoya disease; N/A, not available; RLS, left-to-right shunt; NSAIDs, non-steroidal anti-inflammatory drugs; TIA, transient ischemic attack; ACA, anterior cerebral artery.
Table 1.
Table 2.
TOP
 KHS
KHS