A Practical Approach to Headache in Moyamoya Disease
Article information
Abstract
Moyamoya disease (MMD) is a progressive steno-occlusive cerebrovascular disorder of the intracranial internal carotid arteries characterized by fragile collateral vessel formation. Although ischemic and hemorrhagic strokes are the most widely recognized manifestations of MMD, headaches are common, often disabling, and remain underacknowledged. Epidemiological studies report headache in 17%–85% of MMD patients, with particularly high rates among pediatric patients. Clinically, headache phenotypes are diverse and include migraine-like headaches with or without aura, tension-type, cluster, and hemiplegic variants. These presentations often overlap with primary headache disorders, complicating the diagnosis and sometimes delaying the recognition of underlying MMD. The pathophysiology of MMD-related headaches is multifactorial, involving vascular stenosis, abnormal collateral circulation, altered hemodynamics, and neurogenic inflammation. Chronic hypoperfusion may lower the threshold for cortical spreading depression, contributing to migraine-like or aura-associated symptoms. Surgical revascularization has been reported to alleviate headaches in both pediatric and adult patients, but persistent or new headaches may occur postoperatively, and long-term outcomes remain inconsistent. Management often involves general analgesics such as acetaminophen and non-steroidal anti-inflammatory drugs, but vasoconstrictive agents (e.g., triptans and ergotamines) should be avoided. Lasmiditan, a non-vasoconstrictive 5-HT1F agonist, may represent a safer option for acute treatment, while the efficacy of other pharmacological agents remains unclear due to limited evidence. In conclusion, headaches in MMD are not only a frequent source of disability but also a potential clinical marker of disease activity. Wider recognition of their epidemiology, phenotypes, and mechanisms may improve the diagnosis, guide individualized treatment, and ultimately enhance quality of life for patients.
INTRODUCTION
Moyamoya disease (MMD) is a rare, progressive cerebrovascular condition characterized by chronic stenosis or occlusion of the intracranial internal carotid arteries and the subsequent development of compensatory collateral vessels, creating a distinctive “puff of smoke” appearance on angiography.1 Recent advances in genetics have shed new light on the pathogenesis of MMD. The RNF213 gene, particularly the p.R4810K variant, has been identified as the major susceptibility gene in East Asian populations, with carriers showing a markedly increased risk of MMD development.2 This variant is thought to affect vascular remodeling and angiogenesis, thereby predisposing to steno-occlusive changes of the intracranial arteries. In addition to RNF213, other genes such as GUCY1A3 and ACTA2 have been implicated in Moyamoya syndromes. Variants in GUCY1A3, which encodes a subunit of soluble guanylate cyclase, have been reported in families with Moyamoya angiopathy, while ACTA2 variants, associated with vascular smooth muscle dysfunction, have been described in syndromic cases with aortic disease and cerebral arteriopathy.3,4 Together, these discoveries not only enhance our understanding of disease mechanisms but may also inform diagnostic strategies, risk stratification, and future targeted therapies.
The clinical manifestations of MMD are diverse, with ischemic strokes and transient ischemic attacks (TIAs) being the most recognized symptoms. However, headaches represent a significant and often overlooked aspect of MMD and they can present with a wide spectrum of phenotypes, including migraine (with or without aura), tension-type headaches, and cluster headaches.5,6
These headaches frequently mimic primary headache disorders, complicating diagnosis and potentially delaying timely intervention. Beyond their symptomatic burden, headaches in MMD may signify underlying cerebral hypoperfusion or hemodynamic instability, underscoring their clinical importance. The pathophysiology of headaches in MMD involves complex interactions between vascular stenosis, neurogenic inflammation, and altered hemodynamics, which contribute to the diverse headache presentations observed in MMD.5,6 This review aims to provide a comprehensive understanding of headaches in MMD by synthesizing current knowledge on their epidemiology, clinical characteristics, pathophysiology, and management strategies. By doing so, it seeks to offer practical insights for optimizing diagnosis and treatment, ultimately improving outcomes for patients with MMD.
EPIDEMIOLOGY OF HEADACHES IN MOYAMOYA DISEASE
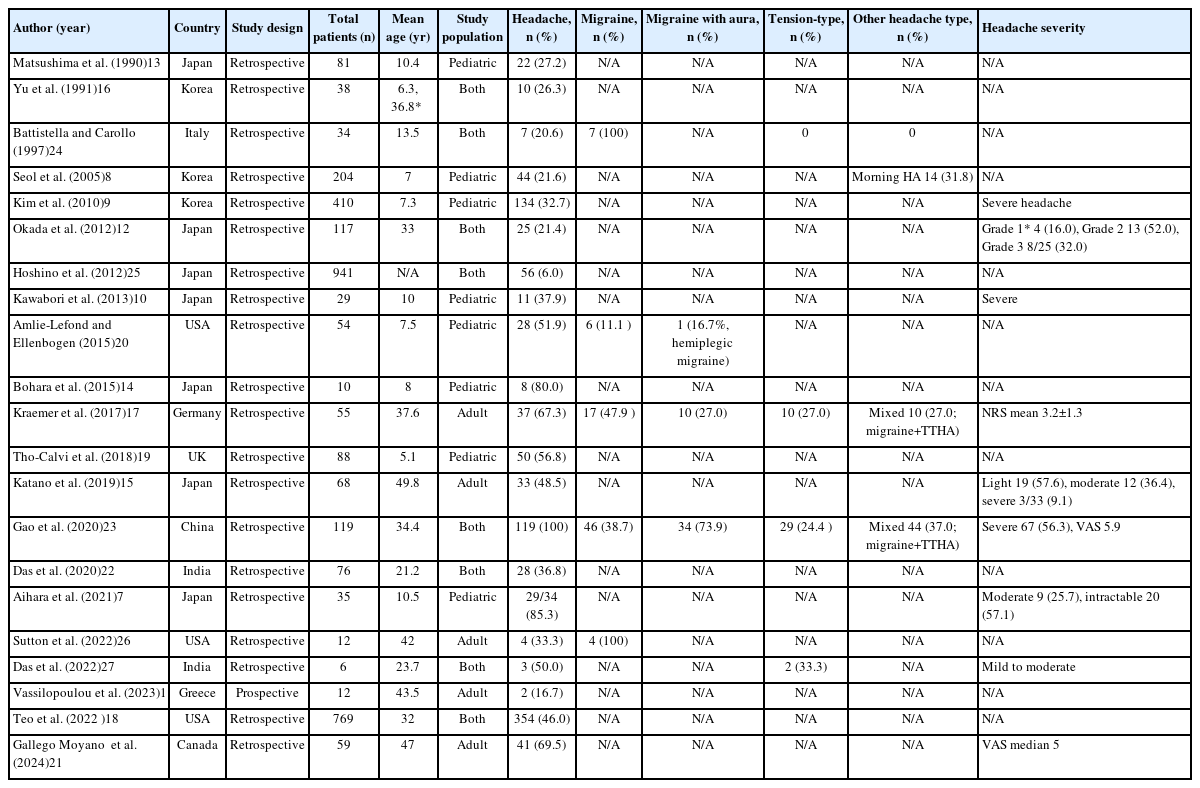
Headaches constitute a prevalent yet frequently overlooked manifestation among individuals diagnosed with MMD. This narrative review synthesizes current evidence on headaches in MMD based on literature retrieved from major databases including PubMed and EMBASE. For epidemiological data (Table 1),7-27 we included observational studies with sufficient sample size and clinical detail, while for clinical characteristics (Table 2),5,28-37 representative case reports and case series were selected to illustrate the diversity of headache manifestations in MMD. A review of multiple observational studies indicates that the reported incidence of headache among patients with MMD ranges from 17% to 85%, with most studies documenting prevalence rates between 30% and 60%.7-11 The substantial variability of headache prevalence is likely attributable to disparities in the demographic characteristics of study cohorts, research methodologies, and the diagnostic criteria utilized in various investigations (Table 1).
Although MMD shows a markedly higher prevalence in East Asian populations compared with Western cohorts, headache manifestations appear to be less strongly influenced by ethnicity. In Japan, reported headache prevalence ranges from 21% to 85%,7,10,12-15 whereas studies from Korea indicate prevalence rates between 22% and 38%.8,9,16 In American and European populations, the occurrence is lower than in Asian groups; however, headaches remain a notable symptom, particularly among pediatric patients (17% to 67%).11,17-20
MMD demonstrates a bimodal age distribution, with incidence peaks during childhood (the first decade of life) and adulthood (around 40 years). Headache is a frequently reported symptom across all age groups, though its prevalence and clinical significance may vary by age. In pediatric patients, headache is often the second most frequent symptom after motor deficits,9,13 with prevalence ranging from 22% to 85% depending on study design, diagnostic criteria, and surgical status.7-9,13,14,19,20 In adults, reported headache prevalence ranges from 17% to 67%.11,15,17,21 While headache has been considered somewhat more common in pediatric MMD than in adults, the evidence for this remains mixed and inconsistent. Several factors may influence these reported differences, including variations in study methodology, differences in headache assessment and definition, and potential age-related differences in symptom reporting. Additionally, the higher frequency of revascularization surgery in pediatric populations may contribute to postoperative headaches, potentially affecting prevalence estimates. Interestingly, when examining patients with similar surgical exposure, Teo et al.18 reported comparable headache rates in children (43%) and adults (47%), while studies by Das et al.22 and Yu et al.16 found higher headache prevalence in adults within the same cohort. These findings highlight the complexity of age-related headache patterns in MMD and underscore the need for additional well-designed prospective studies that account for surgical status, disease stage, and the use of standardized headache assessment tools to better clarify the true relationship between age and headache manifestations in this disease.
Gender differences in headache prevalence among patients with MMD have been less extensively studied and remain inconclusive. While some studies reported a higher prevalence of headache in males compared to females,10,12 Katano et al.15 found the opposite trend, with headaches more frequently reported in females (57%) than in males (32%). These conflicting results are noteworthy given the female predominance of MMD itself and the higher prevalence of primary headache disorders among females in the general population. Further investigation is needed to clarify these gender-specific patterns in MMD-associated headache.
Headache in MMD presents with three distinct temporal patterns: preceding MMD diagnosis, as an initial presenting symptom, and following diagnosis or revascularization surgery. Some patients experience recurrent headaches months to years prior to diagnosis, and the median interval from headache onset to MMD diagnosis is approximately 9.5 months (range, 0–192 months).5 As an initial symptom, headache is reported in 7% to 33% of patients, particularly in pediatric cases.9,13 Headache may also newly develop or persist after diagnosis, especially following revascularization surgery.8,9,17,21 Among patients with preoperative headaches, 64%–100% report improvement following surgery, whereas 6%–16% of those without prior headache experience new-onset headaches postoperatively.8-10,14,17,19,21 In line with this, the International Classification of Headache Disorders, 3rd edition (ICHD-3), formally recognizes “Headache attributed to Moyamoya disease” (code 6.8.2). This category applies to patients with a new or significantly changed headache in close temporal relation to MMD onset or progression, with radiological confirmation of the disease. These temporal patterns underscore the need to consider MMD in the differential diagnosis of new or refractory headaches, especially in younger patients, and highlight the importance of clinical history and neuroimaging in guiding timely diagnosis and tailored management.
CHARACTERISTICS OF HEADACHE IN MOYAMOYA DISEASE
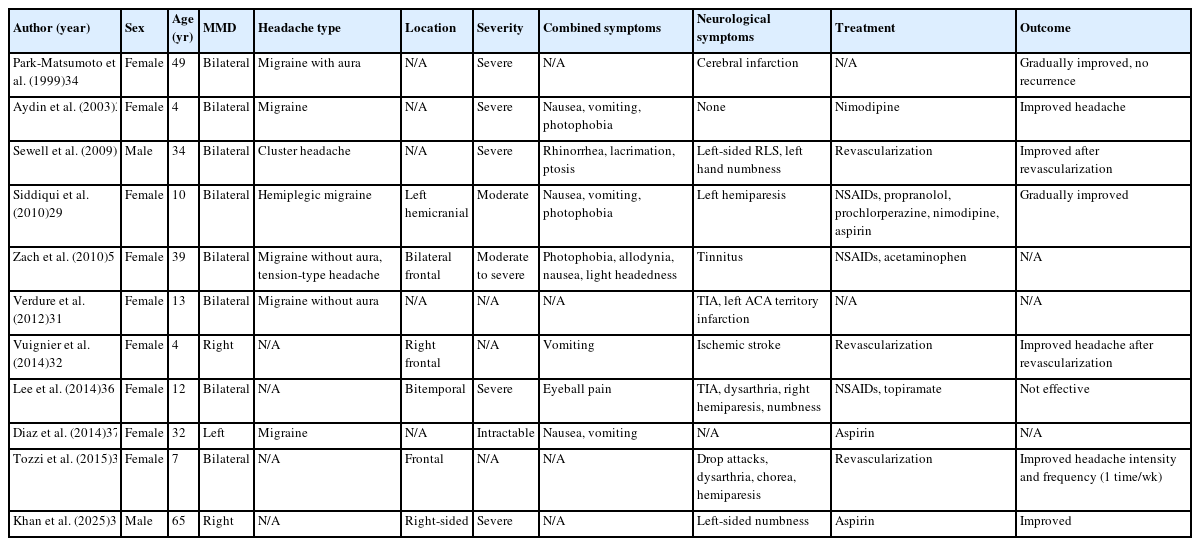
The clinical features of headache in MMD are diverse and often resemble those of primary headache syndromes, but arise from distinct cerebrovascular mechanisms. Migraine-like presentations are particularly common, with several large observational studies reporting migraine prevalence ranging from 39% to 48% among MMD patients with headache.17,23 A distinctive feature is the high prevalence of migrainous aura (27%–74%), with visual, sensory, motor, and speech disturbances reported. This is substantially higher than the 30% aura prevalence typically seen in primary migraine, suggesting that the underlying cerebrovascular pathology in MMD may facilitate cortical spreading depression and aura phenomena. Tension-type headaches affect approximately 25% of patients, while 8%–37% experience mixed headache patterns combining both migraine and tension-type features.17,23 Cluster headache has been documented in rare cases, typically in male patients.28 Hemiplegic migraine variants have also been reported in the literature, particularly in pediatric patients.29
The quality of pain in MMD-associated headaches varies widely. Case studies describe throbbing or pulsatile pain characteristic of migraine, as well as pressing or oppressive pain typical of tension-type headache (Table 2).5,29,30 Occasionally, patients experience thunderclap headaches with sudden, severe onset, which may signal acute changes in cerebral hemodynamics or incipient ischemic events.31
The severity of headaches in MMD generally tends toward the moderate-to-severe range in case series.5 Many observational studies that assessed headache prevalence used criteria emphasizing functional impairment, often classifying headaches as severe if they interfered with daily activities.9,12,23 For instance, Aihara et al.7 reported in a pediatric MMD cohort that 57% of patients experienced intractable headaches, while 26% reported moderate-intensity headaches, highlighting the significant burden of symptoms even in younger populations. These findings underscore the disabling nature of MMD-associated headache and its potential impact on quality of life, especially in cases resistant to conventional analgesic treatment.
The location of headache in MMD patients demonstrates considerable variability. Frontal and temporal regions are most commonly affected, with both unilateral and bilateral distributions frequently reported.10,15,29,32 Importantly, headache laterality does not always consistently align with the side of vascular involvement, and the correspondence appears inconsistent across cases.29 This suggests that pain generation in MMD likely reflects broader hemodynamic or neurogenic mechanisms rather than focal vascular stenosis alone. Duration patterns also vary widely, ranging from several hours to multiple days, with most cases reporting headache episodes lasting less than 24 hours.
Associated symptoms frequently accompany headaches in MMD, enhancing their similarity to primary headache disorders. Nausea and vomiting are common accompanying symptoms, reported in multiple observational and case studies.8,21 Photophobia, phonophobia, and occasionally allodynia are also reported, further mimicking the symptom profile of primary migraine.5,21 In patients with cluster-like presentations, ipsilateral autonomic features such as lacrimation, rhinorrhea, and ptosis may occur, though these appear to be relatively rare.28
A distinctive feature of headache in MMD is its frequent association with neurological symptoms, either concurrent with headache episodes or as separate manifestations. In MMD patients with headache, TIAs, hemiparesis, dysarthria, numbness, and visual disturbances are commonly reported in association with headache episodes (Table 2).28,29,31,33 This pattern of alternating or concurrent headache and focal neurological deficits is strongly suggestive of an underlying cerebrovascular disorder and should prompt consideration of MMD in the differential diagnosis, particularly in young patients without conventional vascular risk factors.
Provocation factors for headache in MMD offer insights into pathophysiological mechanisms. Physical exertion, stress, and straining effort are reported triggers in several cases.5,30,31 Seol et al.8 specifically demonstrated that hyperventilation triggered headache in four patients, three of whom also experienced TIAs, suggesting a shared hemodynamic mechanism involving vasoconstriction and reduced cerebral perfusion. Morning predominance of headache was reported in 31.8% of patients in the same study, potentially reflecting nocturnal hypercapnia or positional changes in cerebral perfusion.
The diagnostic challenge of headache in MMD lies in its phenotypic overlap with primary headache disorders. Several distinguishing features should raise suspicion for underlying MMD, including: (1) atypical age of onset (very young children or young adults without family history of migraine); (2) concurrent or alternating focal neurological deficits; (3) headache triggered by exertion or hyperventilation; (4) refractory headache unresponsive to conventional treatments; and (5) unusual aura phenomena, particularly prolonged or atypical aura symptoms. The presence of these features should prompt consideration of neurovascular imaging, even in patients with otherwise typical-appearing headache presentations (Figure 1).
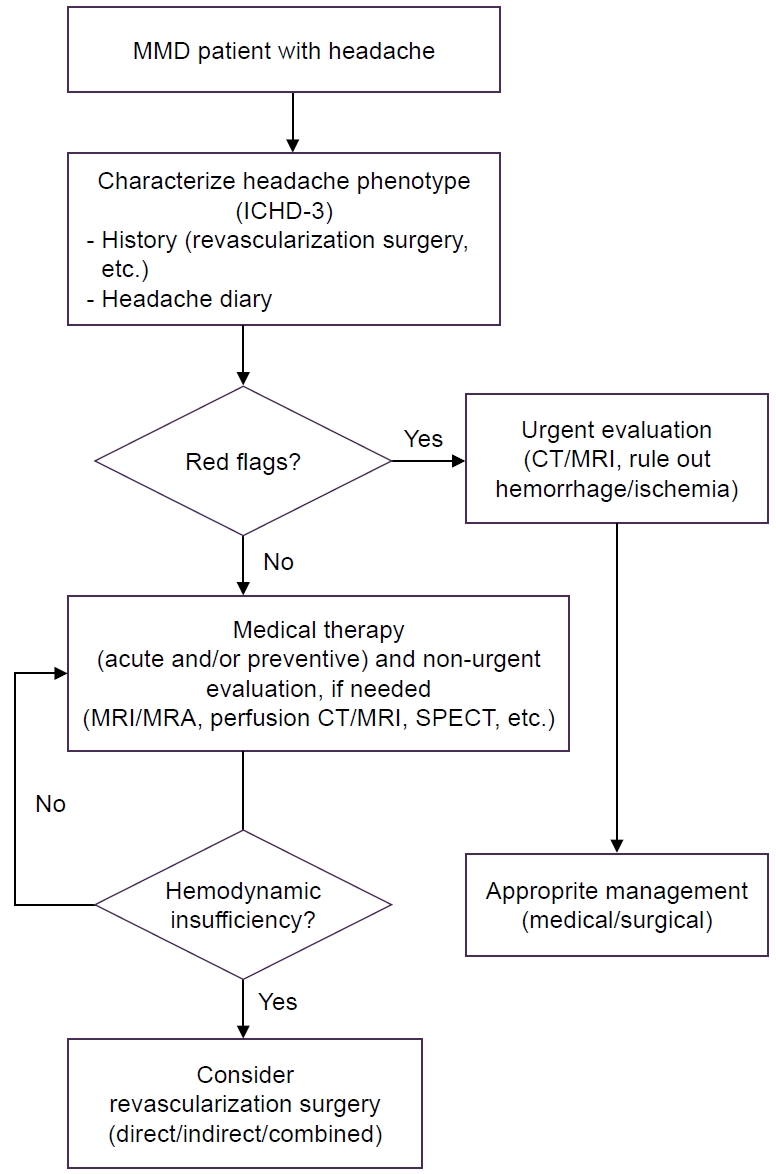
A practical approach to headache associated with Moyamoya disease (MMD). “Red flags” include the presence of sudden focal neurological deficits; the occurrence of thunderclap headache characterized by abrupt and severe pain; the presence of seizures or cognitive changes associated with headache; and new-onset or worsening headache.
ICHD-3, International Classification of Headache Disorders, 3rd edition; CT, computed tomography; MRI, magnetic resonance imaging; MRA, magnetic resonance angiography; SPECT, single-photon emission computed tomography.
PATHOPHYSIOLOGICAL MECHANISMS OF HEADACHES IN MOYAMOYA DISEASE
The underlying pathophysiological mechanisms of headaches in MMD remain poorly understood. These mechanisms are complex and involve both primary and secondary headaches. Several plausible mechanisms have been proposed.
First, nociceptors in cerebral arteries and the dura have been suggested as one of the culprits. MMD is a chronic, progressive steno-occlusive disease characterized by the development of compensatory collateral circulation, particularly involving vasodilation of cortical vessels and small perforators in the skull base. This vasodilation may stimulate dural nociceptors, especially through transdural or leptomeningeal collaterals, and perivascular nociceptors.38 Additionally, cerebral aneurysms are observed in 3%–18% of MMD patients and may occur in major trunks of the circle of Willis or peripheral vessels, including the choroidal, lenticulostriate, meningeal, or moyamoya arteries.39 Enlargement of these aneurysms may lead to activation of vascular nociceptors.
Second, neurogenic inflammation, a form of inflammation initiated by the activation of peripheral sensory nerve fibers (especially nociceptors), may contribute to headache in MMD.40 Stimulation of dural nociceptors via dilated leptomeningeal collaterals could activate and sensitize the trigeminovascular system through the release of calcitonin gene-related peptide (CGRP), substance P, nitric oxide, and pituitary adenylate cyclase-activating polypeptide, resulting in neuroinflammatory responses such as mast cell degranulation, plasma protein extravasation, and vasodilation.6,41-43 This mechanism shares features with the neuroinflammation underlying migraine pathophysiology.5,17
Third, chronic intracranial hypoperfusion and hypoxia may contribute to headaches, as cerebral hypoperfusion is known to lower the threshold for migraine and increase the risk of cortical spreading depression.34,44 Migraine-like headaches are, in fact, the most commonly reported headache type in MMD. Cortical spreading depression triggered by chronic cerebral hypoperfusion may be associated with the high prevalence of migraine with aura in MMD.17,45 Chronic ischemia leads to the overexpression of pro-inflammatory cytokines such as interleukin-1β and tumor necrosis factor-α, as well as vascular endothelial growth factor, which can sensitize perivascular nerves.6,46 Notably, revascularization aimed at improving ischemia and cerebrovascular reserve has been reported to reduce the intensity and frequency of preoperative headaches, regardless of the bypass type (direct or indirect) and age of onset.10,13,21 However, most studies did not specify headache subtypes in detail. Conversely, headaches may also occur after revascularization surgery, often referred to as postoperative headaches. One study evaluating postoperative headaches following direct bypass surgery in adult MMD patients found that postoperative superficial temporal artery (STA) diameter and the rate of postoperative increase in STA diameter were significantly associated with postoperative headaches.15 This suggests that STA dilation may be responsible, as the outer layer of the STA contains nociceptive nerve endings and sensory fibers derived from the trigeminal nerve.
Fourth, headaches—particularly in pediatric MMD patients—may result from platelet aggregation activity secondary to endothelial cell damage. In a study of 35 pediatric patients with ischemic-onset MMD, low-dose aspirin use was associated with an improvement in intractable headaches in 85% of patients within one month after revascularization surgery.7
Finally, it is important to consider that some patients may suffer from coincidental primary headaches such as migraine or tension-type headache, considering the high prevalence of primary headaches in the general population.
MANAGEMENT OF HEADACHES IN MOYAMOYA DISEASE
Migraine-like headaches, either alone or accompanied by tension-type headaches, are the most commonly reported headache types in MMD.23 Treatment strategies for headaches in patients with MMD have not been standardized due to the lack of relevant studies. Although some approaches are derived from the management of migraine or tension-type headaches, they are mostly based on clinical experience and the underlying pathophysiological characteristics of MMD—chronic steno-occlusion with compensatory collateral formation, fragile vascular networks, and medication side effects.47
Medical management of headaches in MMD has not been systematically studied and the most appropriate and effective analgesics for MMD-related headaches remain unknown. From a safety standpoint, general pain relievers such as acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used, with vasoconstrictive drugs typically avoided. However, caution is needed when using NSAIDs due to their potential to increase bleeding risk.
Common migraine medications such as triptans (5-HT1B/1D agonists) and dihydroergotamine should generally be avoided in MMD, particularly in cases with abundant leptomeningeal collaterals, due to their vasoconstrictive effects. Lasmiditan, a selective 5-HT1F receptor agonist that inhibits neuropeptide release and pain transmission in the trigeminovascular system, may serve as a safer alternative for the abortive treatment of migraine-like headaches in MMD, as it does not induce vasoconstriction.48,49 CGRP-targeted therapies, including monoclonal antibodies and CGRP antagonists, have recently emerged as a novel class of agents in migraine treatment. However, given that CGRP acts as a potent vasodilator,50,51 these therapies may not be suitable for MMD patients, as they could inhibit essential compensatory vasodilation.
As mentioned earlier, low-dose aspirin may be more effective in pediatric MMD patients with intractable headaches than analgesics such as NSAIDs or acetaminophen.7 In addition, a nationwide cohort study demonstrated that cilostazol use in patients with MMD was associated with improved survival compared with other antiplatelet agents, likely due to its combined antiplatelet, vasodilatory, and endothelial-protective effects.52 Furthermore, a high-resolution MRI study showed that cilostazol was associated with favorable vessel wall changes and stabilization of disease progression in adult-onset MMD.53 Although direct evidence on headache outcomes is lacking, these findings suggest that cilostazol may alleviate ischemia-related headaches by improving cerebral perfusion and modifying disease course, thus warranting further prospective studies. However, the efficacy of other antiplatelet agents or their combinations remains unclear due to a lack of relevant studies.
For migraine prevention, a range of pharmacological and non-pharmacological strategies can be considered in MMD, particularly when revascularization is not feasible or when headaches persist after surgery. Pharmacological preventives include antidepressants, anti-seizure medications, beta-blockers, calcium channel blockers, and botulinum toxin A.54 In the context of MMD, however, beta-blockers and calcium channel blockers should be prescribed with caution because of their potential to worsen cerebral hypoperfusion. Among these, amitriptyline, topiramate, propranolol, and cyproheptadine are commonly used in pediatric migraine, whereas others, such as botulinum toxin A, are currently approved only for adult chronic migraine prophylaxis. Sodium valproate has also been reported in a pediatric MMD case to markedly reduce hemiplegic migraine–like attacks, highlighting its potential role as a preventive therapy, especially in patients with coexisting seizures.55 Consistent with the Korean Headache Society guideline, valproate remains an established option for migraine prevention, although its safety profile requires careful consideration. Botulinum toxin A, while not specifically studied in MMD, is guideline-endorsed for chronic migraine prophylaxis in adults and may be considered in refractory MMD patients with chronic migraine–like headaches, given its lack of vasoconstrictive or hemodynamic effects. Non-pharmacological interventions- including regular sleep hygiene, dietary changes, adequate hydration, increasing physical activity, and behavior therapy-may be recommended as initial treatment and complement pharmacological therapies to both pediatric and adult MMD patients due to their high safety and tolerability.
Revascularization surgery, irrespective of the technique employed, has been reported to alleviate headaches in both pediatric and adult patients with MMD. Nevertheless, headaches may persist or newly emerge following surgery.9,12 A recent study involving 119 Chinese MMD patients (80.6% underwent surgery; 19.4% received conservative treatment) assessed the impact of surgery on headache outcomes. While preoperative headaches improved in the surgical group, there was no significant difference between the surgical and conservative groups in terms of long-term effects on headache frequency and intensity over a 5-year follow-up.23 These findings suggest that in MMD patients who undergo bypass surgery, the underlying mechanisms of postoperative headaches may be more complex and differ from the preoperative headache mechanisms discussed above. These include postoperative dynamic change of cerebral circulation, stimulation of dural trigeminal nociceptors and neurogenic inflammation following indirect or direct bypass, surgical trauma to intracranial structures caused by craniotomy, and aggravation of dilated collateral vessels in accordance with the disease progression.6,8 Therefore, the primary goal of revascularization should remain the prevention of ischemic and hemorrhagic events rather than headache relief (Figure 1).
BROADER IMPLICATIONS, APPLICATIONS, AND CONCLUSIONS
Headache in MMD represents more than a comorbid symptom—it serves as a potential clinical indicator of hemodynamic compromise and cerebrovascular instability. Recognizing the diverse and often migraine-like headache patterns associated with MMD may improve early detection, particularly in young or otherwise low-risk individuals. This review aims to provide a practical overview of headaches in MMD by synthesizing evidence on their epidemiology, clinical features, underlying mechanisms, and management strategies. Headache is one of the most frequent and disabling symptoms in MMD, particularly in pediatric patients, and can present with diverse phenotypes, including migraine-like, tension-type, and hemiplegic patterns. These overlapping features with primary headaches complicate diagnosis but may serve as an important clinical clue to underlying cerebrovascular disease.
Pathophysiological insights suggest contributions from chronic cerebral hypoperfusion, abnormal collateral circulation, and neurogenic inflammation, highlighting why MMD-related headaches are often severe and refractory to conventional therapies.
Treatment strategies remain empirical and individualized. While analgesics are used cautiously, vasoconstrictive agents such as triptans and ergotamines are generally avoided. Preventive therapies, including commonly used migraine medications, may be considered on a case-by-case basis, complemented by non-pharmacological approaches. Revascularization surgery can improve headaches in some patients, though long-term outcomes are inconsistent, and headache relief should not be the sole indication for surgery.
In conclusion, headaches in MMD are not only a source of disability but also a potential marker of disease activity. Greater awareness of their clinical spectrum and mechanisms can aid earlier recognition, guide more rational management, and improve quality of life for patients. Future studies should adopt prospective designs and standardized headache assessments to establish evidence-based guidelines for treatment.
Notes
AVAILABILITY OF DATA AND MATERIAL
The data presented in this study are available upon reasonable request from the corresponding author.
AUTHOR CONTRIBUTIONS
Conceptualization: JCC; Writing–original draft: MYE, JMJ, JCC; Writing–review & editing: MYE, JMJ, JCC.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING STATEMENT
Not applicable.
ACKNOWLEDGMENTS
Not applicable.